Cryoport Systems’ Temperature-Controlled Supply Chain Blog
Learn more from our expert leaders on the latest industry trends, global news, and company updates.

04/16/2024
Overcoming Industry Obstacles for Cell and Gene Therapy Development
In the rapidly advancing world of cell and gene therapy (CGT) development, finding the proper solution to overcome common industry obstacles is a challenge that can impact entire production timelines.
04/09/2024
National Holiday Facility Closures
As we look to the rest of 2024, Cryoport Systems wants to ensure that you’re up to date on our service schedule.
03/27/2024
Tec4med: The Newest Addition to Cryoport’s Team
Cryoport Systems is a part of a nexus of supply chain solutions companies all under Cryoport, Inc. The other business units are CRYOGENE, CRYOPDP, and MVE Biological Solutions...
02/22/2024
Examining 2023 & 2024: What’s to Come with President & CEO, Mark Sawicki
The cell and gene therapy industry is poised for many changes in 2024. With persistent challenges facing manufacturers and developers such as the retention of experienced talent or a general lack of funding for specialized therapies, the state of what’s to come feels more unsure than it has in recent years.
02/06/2024
Enabling the Outcome with Cryoport Systems
Cryoport Systems entered the market over two decades ago to support the logistics processes of life sciences organizations. As industry demands evolved, our logistics capabilities expanded into a robust platform of supply chain management offerings.
01/18/2024
Ring in the Rebrand: Nasdaq Bell Ringing Ceremony Recording
Cryoport Systems started off the New Year strong with the unveiling of our rebrand, which includes succinct looks for our parent and sibling companies. On January 17th, we were fortunate enough...
12/31/2023
2023 Acquisitions, New Facilities, and A Rebrand
As we close out 2023, we look back on the year and reflect on the variety of ways Cryoport Systems was able to support our biopharma, reproductive medicine, and animal health clients.
12/21/2023
Examining 2023 & 2024: Looking Back with President & CEO, Mark Sawicki
2023 was a year of highs and lows for the cell and gene therapy (CGT) market. The Alliance for Regenerative Medicine reported...
11/14/2023
3 Services Your Biopharmaceutical Supply Chain Partner Should Offer
When it comes to choosing a cell and gene therapy shipping partner, not all providers are created equal.
10/10/2023
The Crucial Role of Planning Ahead in Your Supply Chain Management
In the dynamic and ever-evolving realm of regenerative medicine and advanced therapies, the reliability of your supply chain could be the difference between success and failure.
09/11/2023
4 Capabilities that Support a Superior Condition Monitoring Solution
For over 10 years, Cryoport Systems has been utilizing our near real-time condition monitoring solution that offers precise location information on shipments.
08/16/2023
How Our Requalification Process Maintains Material Integrity
How can you be sure that your supply chain management partner understands the importance of cleanliness in standardized shipping practices?
07/26/2023
A Glance into Our Houston & Morris Plains Global Supply Chain Centers
As cell and gene therapies continue to transform modern medicine, Cryoport Systems evolves its products and solutions to meet the needs of the industry and the patients it serves.
05/31/2023
3 Services Your Biopharmaceutical Supply Chain Partner Should Offer
When it comes to choosing a cell and gene therapy shipping partner, not all providers are created equal.
04/07/2023
Exclusive Q&A with Bruce McAfee, Director of BioServices Commercial Support
Cryoport Systems has extended its expertise in temperature-controlled supply chain solutions with vein-to-vein BioServices offerings.
03/07/2023
Innovation and Risk Mitigation are Priorities for Cell&Co BioServices
At Cell&Co BioServices, a Cryoport Systems company, new product innovations are not adopted without significant testing. Recently our team dedicated 3 months to the creation and certification of a new type of cryogenic freezer technology.
02/28/2023
State of the Industry: 2023 Predictions with Dr. Mark Sawicki
Cryoport Systems has grown to become a leader in the cell and gene therapy industry since its founding in 1999.
12/13/2022
Documentary: Cell & Gene Therapy – Advancing The Next Generation of Pharmaceuticals
In this video documentary, American Pharmaceutical Review and Pharmaceutical Outsourcing magazine spoke with Cryoport Systems' CEO, Mark Sawicki...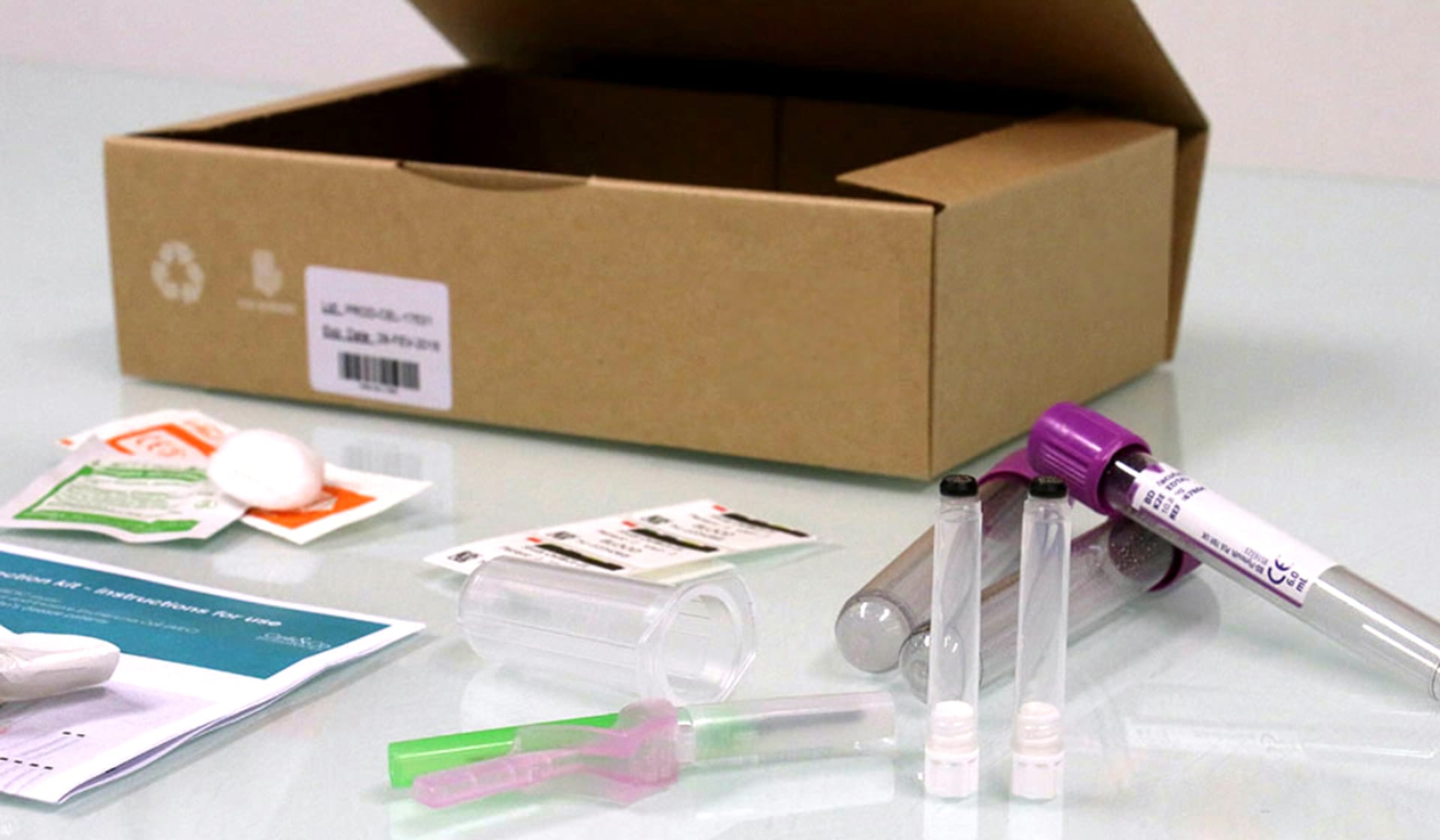
11/30/2022
Through Our Newly Launched Global Supply Chain Centers, Cryoport Systems Now Offers Kit Production
Cryoport combines its expertise in temperature-controlled supply chain solutions to develop a comprehensive offering of vein-to-vein services, one of which includes Kit Production.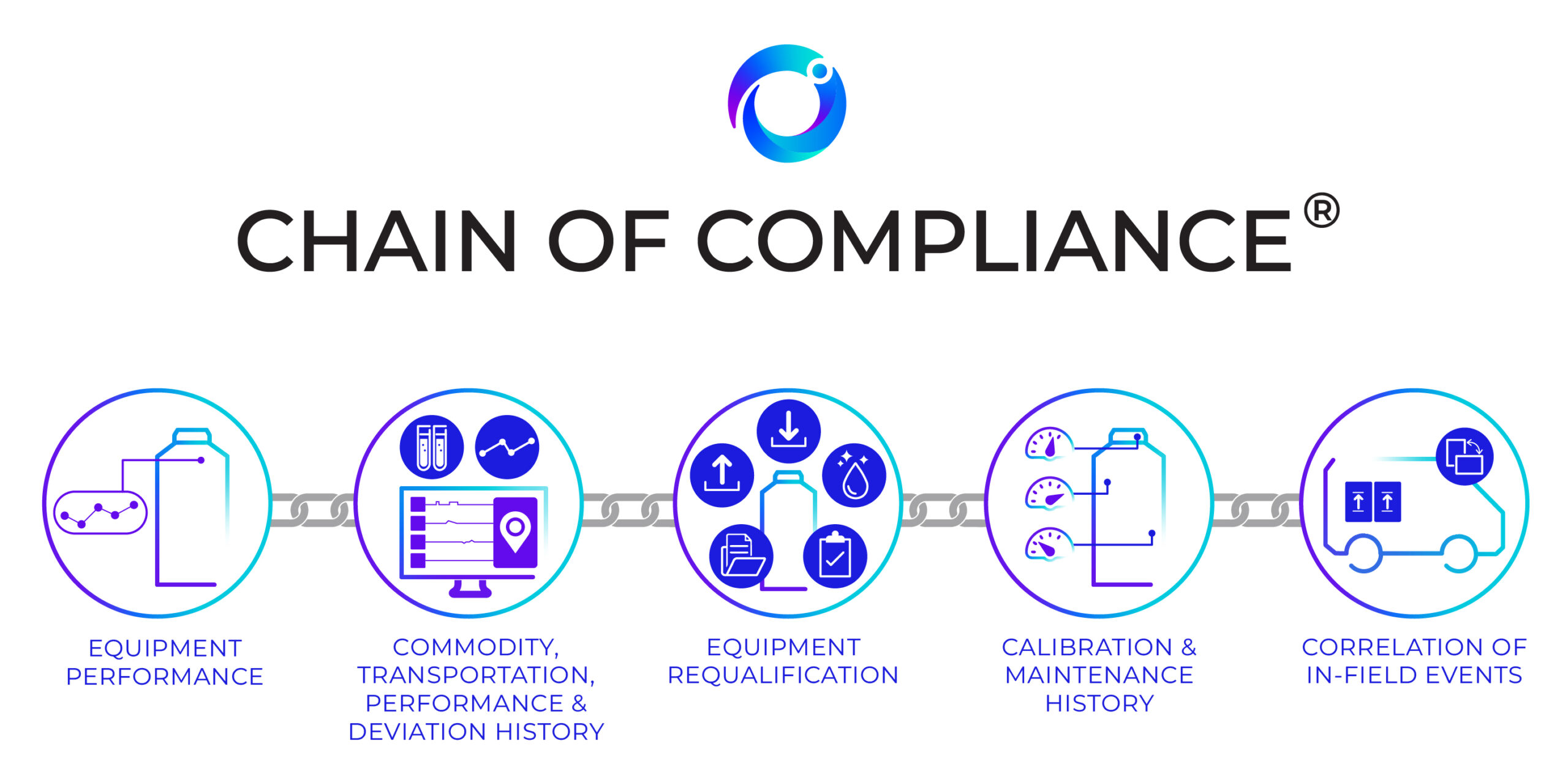
10/19/2022
Cryoport’s Chain of Compliance®: Collects, Interprets, and Leverages Comprehensive Data to Enable a Significantly Smarter Supply Chain
Companies vying to be the first to market with breakthrough treatments have a great deal riding on the success of their efforts.
10/13/2022
Cryoshuttle® Local Delivery and Pickup: Now Serving More Areas
Many biopharmaceutical companies, academic institutions, and manufacturing sites often need to move their valuable life-saving therapies and research materials locally for a variety of reasons...
08/29/2022
The Cryoport Way: A Leap Forward in Standardizing the Regenerative Medicine Supply Chain
As cell and gene therapies are rapidly moving beyond clinical trials and into commercial markets, the safe and effective delivery of these life-saving treatments needs to be a priority for advanced therapy developers.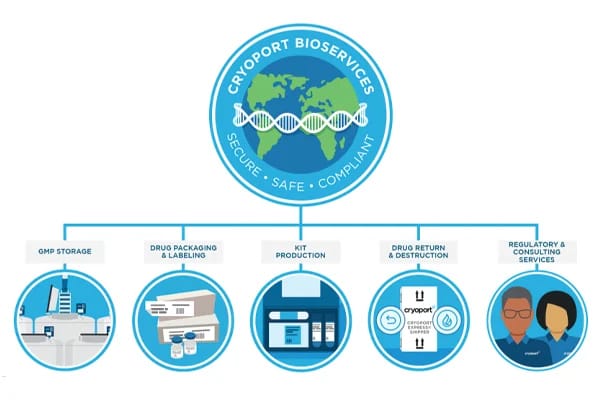
08/22/2022
Cryoport Systems’ Global BioServices: Secondary Packaging and Labeling Solutions
Cryoport Systems’ highly anticipated Global Supply Chain Centers have been operational for a few months, and customers continue to move biopharmaceutical materials into our Houston, Texas...
07/20/2022
The Untapped Potential for Cord Blood as Allogeneic Starting Material
In support of Cord Blood Awareness Month, Cryoport Systems’ partner, Be The Match BioTherapies®, led a webinar focused on the untapped potential and benefits of cord blood for prospective therapies.
06/06/2022
Cryoport Systems Global Supply Chain Center: Ready for Launch!
We are quickly approaching the Grand Opening of our first two Global Supply Chain Centers in Houston, TX on June 14th and Morris Plains, NJ on June 16th, 2022.
05/19/2022
New Product Launch: The Latest Enhancements to Cryoport Express® Standard & Combo Cryogenic Shippers
At Cryoport Systems, we are continuously striving to align with the latest science and technology advancements in our products and services, integrating the most advanced packaging, informatics...
02/11/2022