


Enabling the Outcome™
Global experts in temperature-controlled supply chain management for critical materials.
Cryoport Systems is your strategic partner for comprehensive solutions that protect high-value commodities throughout the supply chain.
Shipping Systems
Cryoport Systems’ ISO 21973-compliant shipping systems support expanded temperature ranges.
Our patented shipping systems safely deliver products, therapies, and treatments that require temperature-controlled management.
Transportation
Logistics services to meet the transportation needs of a wide range of materials.
Providing the safe transportation of commodities at cryogenic, ultra-cold (dry ice), refrigerated, and controlled room temperature.
Cryopreservation
Cryoport Systems IntegriCell® cryopreservation services to support advanced therapies.
IntegriCell cryopreservation services standardize protocols, processes, and equipment to support consistent, high-quality leukapheresis starting material.
BioServices & Biostorage
Expanded biomaterials management capabilities encompassing every aspect of the supply chain.
Integrated BioServices solutions directly support the intricacies of advanced therapies from clinical trials to commercial material management.
Consulting & Advisory
Tailored solutions that deliver value and insight for your complex temperature-sensitive supply chain.
Complex biologics and cell & gene therapies need a robust, temperature-sensitive supply chain. Our team of experts can help de-risk your logistics.
Find your complete solution.
Cryoport Systems provides a comprehensive range of integrated temperature-controlled supply chain solutions. Explore our offerings by clicking through the drop down below.
Select solution:

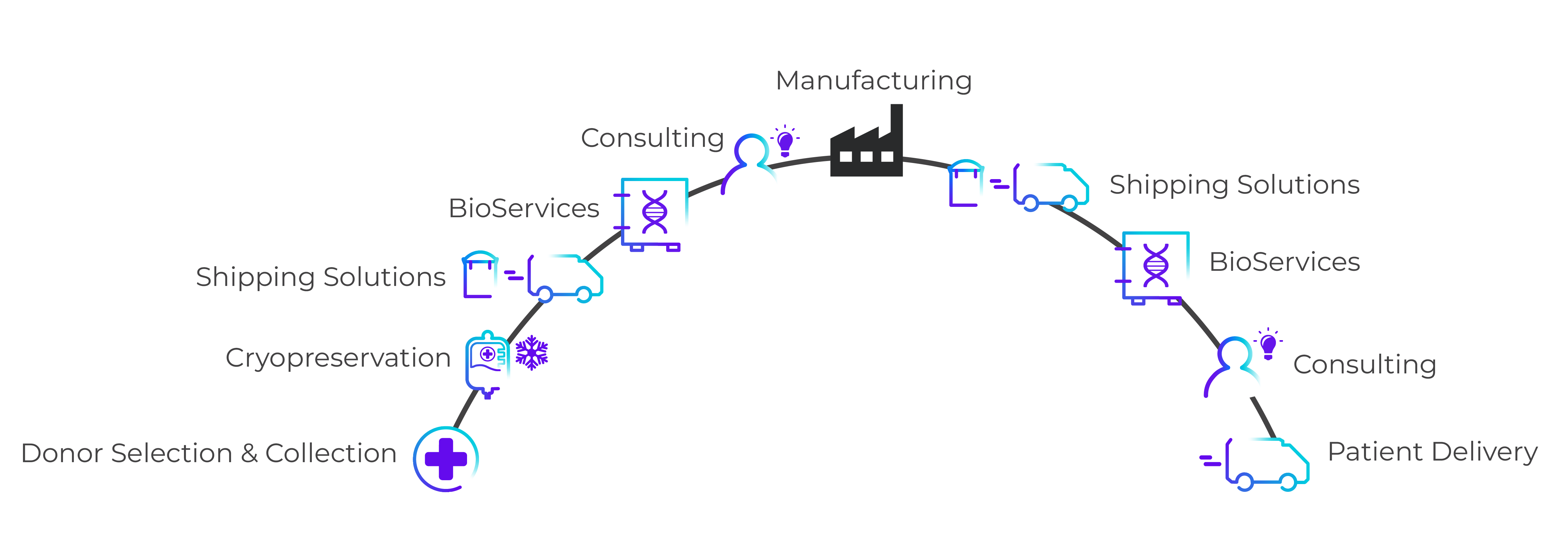
Robust supply chain management solutions and trusted logistics in a comprehensive, end-to-end platform.
Comprehensive Supply Chain Management
Supporting your end-to-end needs, with customizable services to support supply chain management upstream and downstream of manufacturing.
- Shipping Solutions
Temperature-controlled packaging solutions.
Learn More >> - Transportation
Transportation of commodities at various temperatures.
Learn More >> - Cryopreservation
Consistent, high-quality leukapheresis starting material.
Learn More >> - BioServices & Biostorage
Integrated solutions to support advanced therapies.
Learn More >> - Consulting
Our team of experts can help de-risk your logistics.
Learn More >>
Cryoport Systems is Enabling the Outcome™ through our unparalleled supply chain platform.
Offering a comprehensive scope of robust supply chain management solutions and trusted logistics.
Cryoport Systems was established by a team of doctors dedicated to serving the life sciences through expert logistics support. Today, our partnership capabilities have evolved beyond logistics into a robust platform of supply chain solutions that supports every stage and phase of the life sciences research, development, and manufacturing process. We are a standalone vendor that can support all aspects of the temperature-controlled supply chain.
LEARN MORE ABOUT HOW CRYOPORT SYSTEMS IS ENABLING THE OUTCOME™.
Our robust quality standards ensure companies meet all critical supply chain regulations.
Learn more about our efforts to standardize the life sciences supply chain.
Supporting certainty through our risk-mitigating supply chain expertise — one patient, one therapy, one product at a time.
Temperature-controlled Supply Chain Management for Critical Materials
At Cryoport Systems, our people, innovative solutions, and leading technologies go above and beyond to help deliver certainty throughout the end-to-end supply chain.
Our robust quality standards ensure companies meet all critical supply chain regulations, and our teams of specialists alongside our innovative platform of industry-leading solutions enable our clients’ journeys from initial research to global commercialization. We offer comprehensive solutions specifically designed for various biopharmaceutical, animal health, and reproductive medicine applications.
-
Cell Therapy
-
Gene Therapy
-
Biologics
-
Animal Health
-
Reproductive Medicine
Put your TRUST in Cryoport Systems – the leading partner in temperature-controlled supply chain logistics for more than two decades.
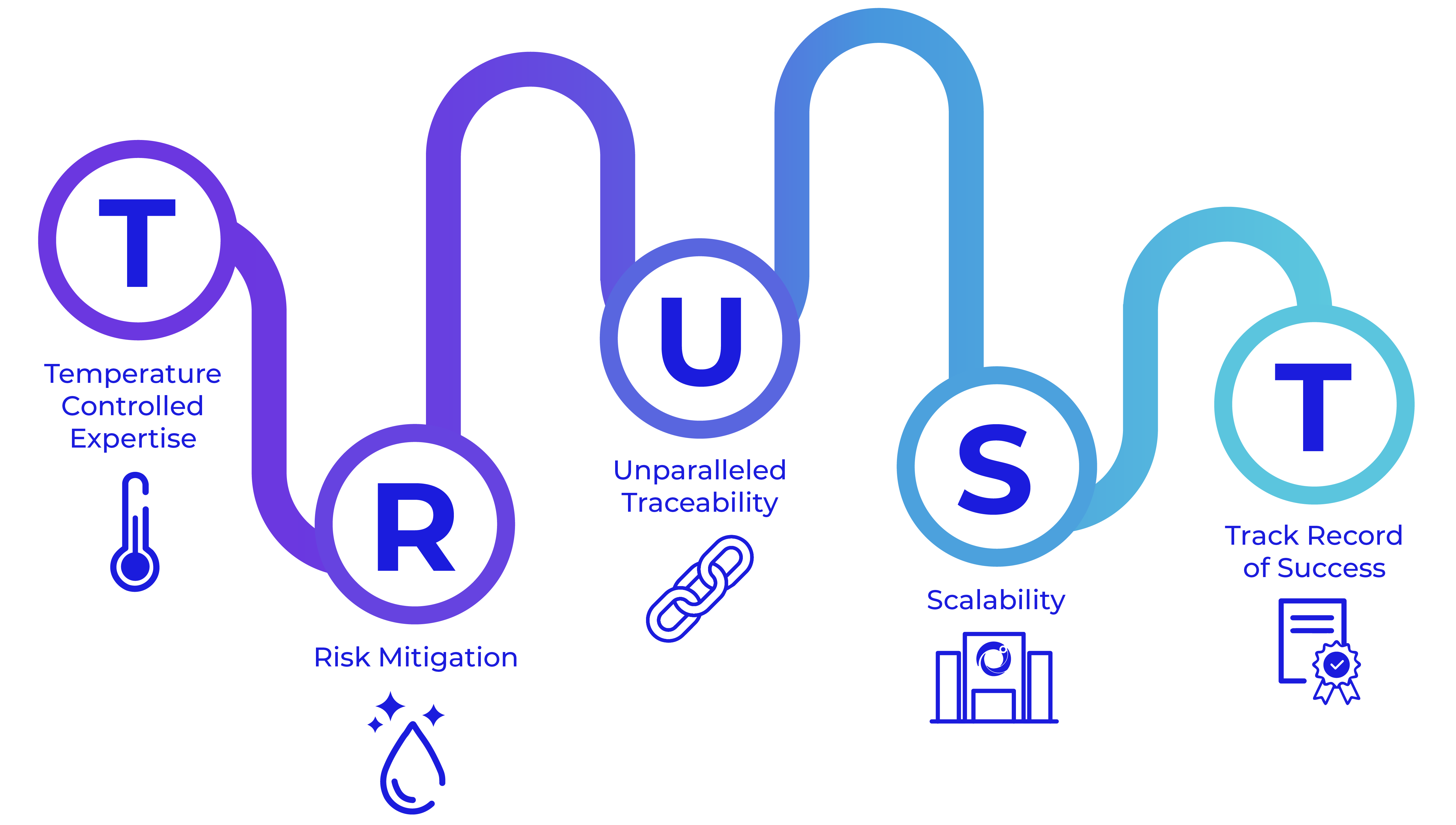
Experience You Can TRUST
We are a global leader and trusted provider of integrated temperature-controlled supply chain solutions.
We lead the way in temperature-controlled expertise for supply chain support. From our proprietary Veri-Clean® process to our requalification procedures, our consistent risk mitigation means we are the only provider to requalify our shippers – every shipment, every time. We stand at the forefront with unparalleled traceability thanks to our Chain of Compliance®. We offer scalability with an expanded depth of services, such as BioServices and biostorage, Consulting Services, and cryopreservation, that add certainty to your supply chain. With 650+ clinical trials supported and more than half a million successful shipments of sensitive and often irreplaceable materials across the globe, you can count on our track record of success that spans decades.
What our clients say about Cryoport Systems.
“I called Cryoport Systems after-hours for information about a shipment I needed to schedule and received absolutely outstanding customer service. Cryoport Systems provided me with a lot of helpful information and great advice. It's rare and refreshing to receive such great service.”
“Not only did my cells arrive safely, Cryoport Systems’ service was professional and efficient. I highly recommend Cryoport Systems.”
“Working with Cryoport Systems was a truly remarkable difference compared to my experience with other companies. With the previous shipping company I was working with, it took almost three months to establish a ‘deep frozen account’ and required numerous hoop-jumping shenanigans only to ultimately be informed that the company could not arrange a shipment to or from Israel, which they were informed from the start was our desired shipping source.”
“Our organization is more than impressed by our experience with Cryoport Systems. We feel confident our material is being well-managed throughout each step in the supply chain. Cryoport Systems provides extraordinary customer service. Continuously, they not only meet but exceed our expectations. Late yesterday afternoon, Cryoport Systems worked tirelessly to coordinate a shipment to Japan, and today seemed to effortlessly manage a late-day return. It is a pleasure working with Cryoport Systems.”
“I want to thank everyone at Cryoport Systems who was involved in the rapid recovery of the missing Cryoport Systems shipper containing a patient’s tumor tissue. By promptly locating the shipper and recharging it while in transit, the patient was able to receive the vaccine manufactured from this tissue on time. We are so very appreciative of Cryoport Systems’ customer service. Without the services that Cryoport Systems provides, our clinical trials would not be available to patients."