Supporting Every Phase of Development

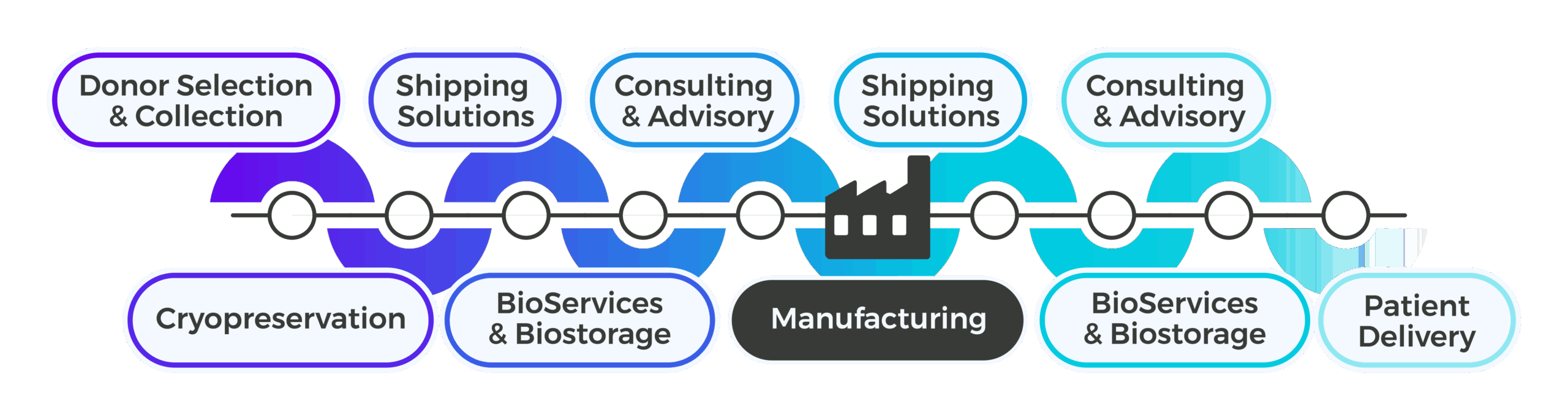
Integrated Supply Chain Support by Development Phase
Tailored solutions for every milestone in your therapy’s journey
Every phase of biotherapeutic development brings unique challenges, and Cryoport Systems is built to meet them. With end-to-end support spanning temperature-controlled logistics, biostorage, kit production, drug packaging and labeling, and consulting services, Cryoport Systems delivers comprehensive capabilities to support the entire lifecycle of advanced therapies. In pre-clinical research, speed and precision are critical to advancing promising science, and our solutions safeguard early-stage materials while streamlining workflows. Phase I trials demand flexibility and compliance as programs scale from research to human studies, and we provide the logistics and supply chain infrastructure that adapts to evolving requirements. Phase II introduces complexity with multi-site coordination and increased regulatory oversight, and our integrated platform ensures consistency and transparency across geographies. By Phase III, global distribution and risk mitigation become paramount, and we deliver validated processes and continuous monitoring to protect product integrity at scale. Commercialization requires a robust, compliant supply chain that can support sustained demand, and our global network and integrated BioServices alongside our global footprint provide exactly that.
With more than two decades of experience and over one million successful shipments, Cryoport Systems has proven expertise in navigating these transitions. Our end-to-end solutions combine advanced logistics, biostorage, kit production, and regulatory support to reduce risk and accelerate timelines. At every milestone, we deliver confidence, compliance, and continuity to Enable the Outcome™ for your program and the patients you serve.
Biopharma Supply Chain Solutions
Scalable, end-to-end support for therapeutic development at every stage
Cryoport Systems provides comprehensive supply chain solutions for biopharma programs, supporting every phase of development from pre-clinical research to global commercialization. Our integrated platform combines advanced logistics with cryopreservation, BioServices and biostorage, consulting and advisory support, and industry-leading quality and compliance. This holistic approach reduces risk and accelerates timelines while ensuring compliance across borders. We understand the unique challenges of cell therapy, gene therapy, and biologics programs and deliver tailored solutions that safeguard product integrity.
Continuous monitoring and ISO-certified processes provide transparency and assurance throughout the lifecycle. Our global infrastructure enables efficient coordination across the entire network of clinical sites, manufacturing facilities, and commercial distribution channels, supported by the industry’s largest wholly-owned fleet of custom-engineered shipping systems and backed by robust, risk-mitigating quality standards. By partnering with sponsors, CROs, and CDMOs alike, we help streamline operations and maintain quality standards. With proven expertise and a track record of success that spans two decades and more than a million successful shipments, Cryoport Systems is the trusted partner for biopharma organizations worldwide.
Support for Pre-Clinical Developers
Start strong by building a scalable, compliant supply chain from day one
Pre-clinical development is the start of your therapeutic’s journey, and it’s where the foundational decisions you make today can impact the future success of tomorrow. At this stage, uncertainty often surrounds process design as you consider regulatory expectations and scalability. Cryoport Systems transforms that uncertainty into confidence by partnering with you early to establish GMP-ready, globally scalable workflows and support.
Our integrated, end-to-end supply chain platform ensures your materials are protected and compliant, prepared for seamless progression into Phase I clinical trials and beyond. Within a single-vendor relationship, you eliminate fragmentation and reduce operational burden, which frees your team to focus on science while we manage the complexity of your supply chain. From defining a standardized cryopreservation workflow to implementing risk-based strategies, we help you build clinical-grade habits early, ensuring reproducibility and uninterrupted supply chain assurance. Beginning your partnership with Cryoport Systems from these early, pre-clinical stages sets you up for success across every phase of development.
Support for Phase I Developers
Secure your first-in-human trials with scalable, compliant supply chain support
Phase I trials mark a critical milestone as your therapy moves from concept to patient care, a milestone that means the margin for error disappears. Every shipment, every process, every decision… everything you do needs to protect patient safety and uphold regulatory standards. Cryoport Systems partners with you from the earliest stages to deliver a supply chain that is IND-ready and globally scalable, designed now for continuity into later phases. Our integrated, end-to-end supply chain platform eliminates fragmentation and reduces operational burden, allowing you to focus on science while we manage complexity.
With fully-owned shipping fleets, integrated condition monitoring, and ISO 21973-certified processes, we ensure your materials are protected across every mile. From cryopreservation of leukapheresis-derived starting material to custom kit building for sample collection and manufacturing, our solutions standardize workflows and mitigate risk. By working with Cryoport Systems, you gain a partner who understands the stakes and delivers confidence for your first patients and every milestone ahead.
Support for Phase II Developers
Scale with confidence by building a global-ready supply chain for expanding trials
Phase II trials represent a critical inflection point for advanced therapies. As patient populations grow and trial complexity increases, the need for a scalable and compliant supply chain that is globally integrated becomes urgent. Cryoport Systems partners with you to ensure that your Phase II program is built for continuity into Phase III and commercialization without costly revalidation or vendor switches.
Our single-vendor supply chain platform approach integrates key services like cryopreservation, BioServices and biostorage, validated logistics, and consulting and advisory services under one framework, reducing operational burden and risk. With ISO 21973-certified processes, Chain of Compliance® tracking, and integrated condition monitoring, we deliver transparency and audit-ready documentation for every shipment. From collection and manufacturing kit standardization to global distribution strategies, our solutions anticipate future demands while protecting patient safety today. By choosing to partner with Cryoport Systems, you secure a supply chain that scales seamlessly and positions your therapy for success across every milestone.
Support for Phase III Developers
Partner with Cryoport Systems to streamline complexity and safeguard your pivotal trials with an end-to-end supply chain platform built for scalability
Phase III trials represent the most demanding stage of clinical development, where operational complexity and regulatory scrutiny reach their peak. Sponsors face the challenge of scaling patient populations, onboarding multiple global sites, and maintaining absolute consistency and compliance across every process. At this stage, fragmented supply chains and vendor transitions can introduce costly delays and compliance risks that jeopardize timelines and BLA approval. Cryoport Systems eliminates these risks through a single-vendor partnership that integrates every critical component of your supply chain under one cohesive platform.
Our solutions are designed to anticipate downstream needs, ensuring your Phase III operations transition seamlessly into commercialization without rework or disruption. With standardized processes across our global network and ISO 21973-certified workflows, we deliver reproducibility and audit-ready documentation with risk mitigation at scale. By partnering with Cryoport Systems, you gain a strategic ally committed to protecting patient safety and accelerating timelines, securing your commercial runway.
Support for Commercial Developers
Partner with Cryoport Systems to deliver therapies worldwide with compliance and confidence. Every dose, every patient, every time.
Commercialization is the moment where precision meets scale. After years of trials and regulatory hurdles, your therapy is finally ready for market, but the complexity of widescale (and oftentimes global) distribution introduces new risks. At this stage, the stakes are higher than ever. Every shipment represents a patient waiting for a life-changing therapy, and any disruption can lead to costly delays or compounding downstream impact. Sponsors need a supply chain partner who can deliver global scalability without sacrificing compliance or reliability, mitigating risks like shipping delays, customs issues, and vendor fragmentation.
Cryoport Systems provides that assurance through our single-vendor supply chain platform that integrates logistics, BioServices and biostorage, cryopreservation, and consulting under one cohesive end-to-end framework. Our solutions are designed to anticipate challenges and mitigate risk, maintaining continuity from API to patient. With our built-in geographic redundancies, validated shipping systems, ISO-certified workflows, and track record of success that spans decades, we ensure your commercial supply chain operates with precision and resilience. With contingency planning built into every process, we provide resilience against disruptions and maintain compliance across every lane.

Cell Therapy Support
Cryoport Systems delivers tailored solutions for cell therapy programs, ensuring compliance and product integrity at every stage.

Gene Therapy Support
Our platform safeguards viral vectors and genetic materials throughout development and commercialization.

Biologics Support
We provide validated processes and global reach to support biologics programs worldwide with risk-mitigating solutions for every phase.




