IntegriCell™ Cryopreservation
IntegriCell™ Cryopreservation Services from Cryoport Systems
Ensuring unmatched quality, consistency, and reliability for advanced cell therapies
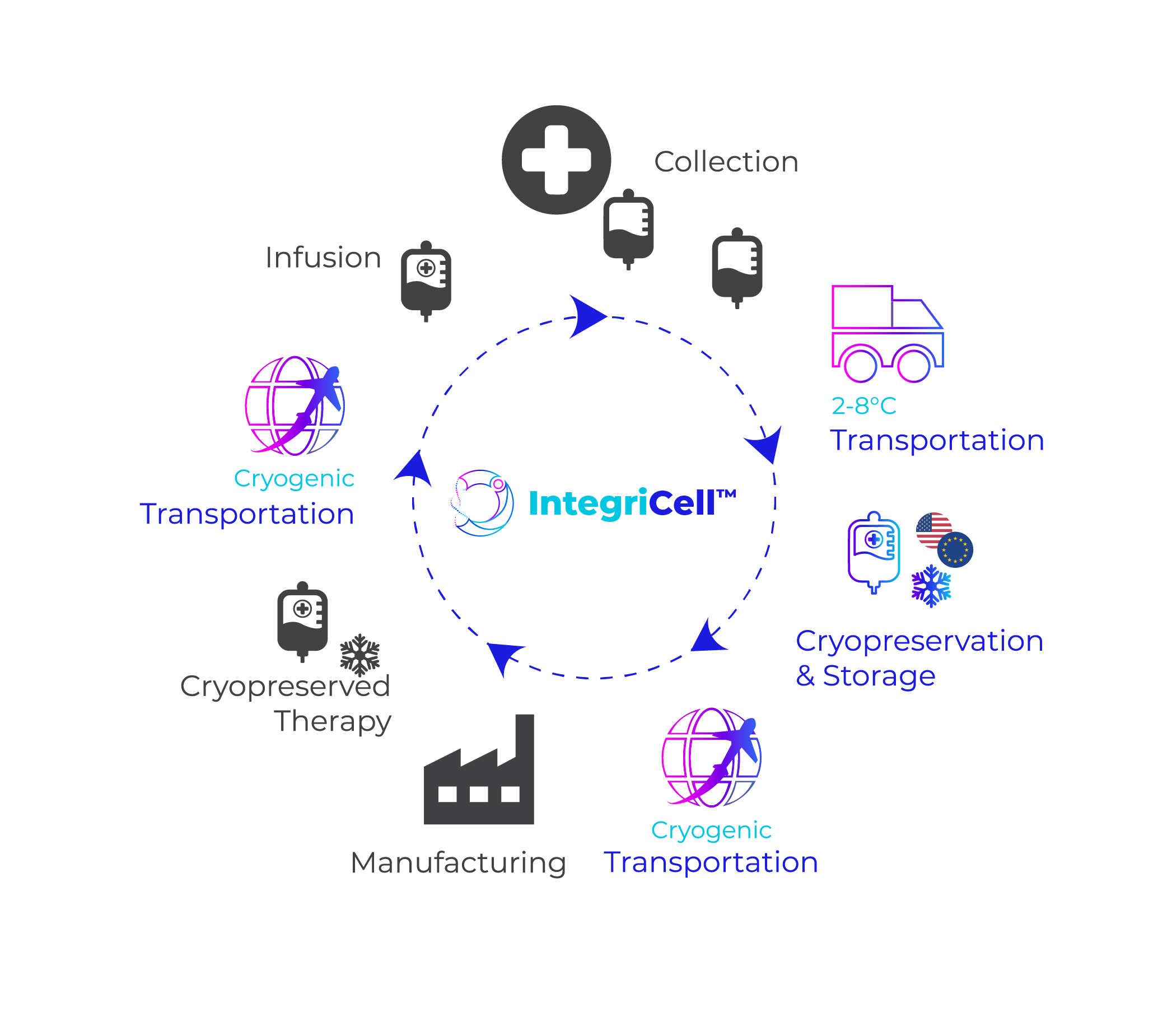
IntegriCell™ cryopreservation services from Cryoport Systems optimize leukapheresis-derived cell therapies by enhancing the safety, quality, and viability of manufacture-ready cryopreserved leukopaks. Our state-of-the-art cryopreservation process, integrated with our end-to-end global temperature-controlled supply chain platform, provides unmatched reliability, efficiency, and scope. Our standardized protocols meet the highest compliance standards, ensuring consistent quality and addressing product stability issues associated with fresh donor-derived cellular material for an optimized approach that enhances manufacturing efficiency and streamlines operations.
Delivering High-Quality Cryopreservation Services for Cell-Based Therapies
Ensuring consistency, compliance, and cost-effective solutions through advanced cryopreservation techniques
IntegriCell™ cryopreservation services are designed to deliver consistent, compliant, high-quality leukapheresis starting material essential for cell-based therapies. Our services ensure that every batch meets the stringent standards required for successful therapeutic outcomes, significantly reducing variability and enhancing reliability. By leveraging advanced cryopreservation techniques and rigorous quality control measures, IntegriCell™ maintains the integrity and viability of leukapheresis samples, ensuring that the starting material is always of the highest possible quality.
Our integrated approach not only prioritizes quality but also aims to minimize risk and costs associated with the cell therapy supply chain. By streamlining processes and incorporating standardized protocols, IntegriCell™ mitigates potential sources of error and contamination, providing a safer, more reliable pathway from collection to final delivery. This comprehensive integration extends across our entire global network, ensuring that each step of the process is meticulously managed and monitored. By ensuring the availability of high-quality, manufacture-ready cryopreserved leukopaks, we enable proactive planning and scaling of cell therapy production processes to support the timely and cost-effective development of advanced cell therapies.
Cellular Viability and Process Optimization
IntegriCell™ cryopreservation services are designed to maximize cellular viability and optimize the entire cryopreservation process. Utilizing state-of-the-art cryopreservation protocols, we ensure that the quality of cellular material is preserved (ideally within the critical 24-hour window post-collection). This approach is crucial for maintaining the integrity and effectiveness of manufacture-ready cryopreserved leukapheresis material, which forms the backbone of advanced cell therapies. Our commitment to high standards means that every step of the process is carefully managed to maximize cellular quality.
A key component of our process is the use of a GMP-compliant, closed automated system, which significantly reduces the risk of contamination and the associated quality variations that can arise from human intervention. This closed automation ensures that each batch of leukapheresis material is handled consistently, minimizing the potential for errors and enhancing overall reliability. By eliminating the variables introduced by manual handling, we achieve a higher level of standardization and quality control, which is essential for producing consistent cell therapy products.
Our approach extends the shelf life of cryopreserved starting material, allowing for the decoupling of collection from drug product production and manufacturing. Cryopreserving leukapheresis material within 24 hours post-collection ensures that the quality of the starting material is maintained over time, providing flexibility and efficiency in the manufacturing process. This capability not only supports better planning and resource allocation but also reduces costs and mitigates risks, ultimately leading to more successful outcomes in cell therapy development and delivery.
Standardization for Consistent Quality
Standardized protocols are at the core of IntegriCell™ cryopreservation services, ensuring the consistency and quality of manufacture-ready cryopreserved leukopaks across our global network. By implementing uniform procedures across the cryopreservation process, we achieve unparalleled reliability. This dedication to standardization allows us to provide high-quality materials that meet the stringent requirements of cell-based therapies, supporting successful clinical and commercial applications worldwide.
Our experienced team, with over 25 years of expertise in cryopreservation, plays a crucial role in maintaining these high standards. Their deep understanding of cryopreservation science and technology ensures that every process is created and implemented with precision and care. By adhering to strict compliance standards, our team guarantees uniformity and reliability in every cryopreservation service, which is essential for producing consistent, high-quality cryopreserved leukapheresis material.
Global standardization further reduces variability and enhances the consistency of our cryopreserved products. By applying the same rigorous cryopreservation processes across all IntegriCell™ locations and facilities, we ensure that every manufacture-ready cryopreserved leukopak we produce meets our exacting standards. This approach minimizes the risk of discrepancies and variations, providing our clients with the confidence that their materials will perform consistently, regardless of where they are processed or which Cryoport Systems facility they are working with. Through this comprehensive standardization, IntegriCell™ delivers the exceptionally reliable and high-quality cryopreserved leukopaks that are essential for producing cell therapies.
Comprehensive Technology Transfer Services
Cryoport Systems’ IntegriCell™ cryopreservation services play a pivotal role in ensuring a smooth and efficient technology transfer for cell and gene therapy developers. Leveraging decades of expertise, our multidisciplinary team works closely with clients to establish and refine cryopreservation processes, facilitating a seamless transition into clinical trials or scaled manufacturing operations.
Our tech transfer process is built on aligning with each client’s unique needs. Led by our experienced MSAT team and working closely with project management, technical operations, analytical, and quality assurance teams, we conduct a thorough evaluation of processes and supporting documentation. This collaborative approach ensures that all procedures are tailored and optimized for scalability, while maintaining compliance and minimizing variability.
By integrating cryopreservation with our state-of-the-art logistics infrastructure, Cryoport Systems enables cell therapy developers to preserve and deliver manufacture-ready cryopreserved leukopaks with precision and consistency. This integration enhances manufacturing flexibility, streamlines timelines, and supports uninterrupted progress from development to commercialization. IntegriCell™ provides the expertise, infrastructure, and operational excellence needed to enable successful tech transfer for advanced therapies.
Maximizing Manufacturing Efficiency
IntegriCell™ cryopreservation services significantly enhance manufacturing slot utilization, driving cost savings and maximizing product revenue for therapy developers. By offering manufacture-ready cryopreserved leukopaks, we provide a level of flexibility and reliability that is crucial for optimizing production schedules. This flexibility allows therapy developers to better align their manufacturing processes with their overall production goals, ultimately improving efficiency and throughput.
One of the key benefits of IntegriCell™ cryopreservation is the scheduling flexibility it offers. Unlike fresh, donor-derived cellular materials, which must be used within a narrow time frame, cryopreserved leukopaks can be stored and utilized as needed. This allows manufacturing to be initiated up to seven days a week, providing therapy developers with greater control over their production timelines. By alleviating the reactive time sensitivity associated with fresh materials, our cryopreservation services contribute to more efficient and predictable manufacturing operations.
Additionally, IntegriCell™ cryopreservation services help reduce backlogs and logistical challenges that can impede manufacturing success. With the ability to store and utilize cryopreserved materials as needed, advanced therapy developers can better manage their supply chain and production schedules, decreasing the risk of delays and disruptions. This results in enhanced manufacturing success rates and a more streamlined process from start to finish. By maximizing manufacturing efficiency, IntegriCell™ cryopreservation services support the timely and cost-effective development of cell-based therapies.
Global Coverage & Support
Cryoport Systems’ IntegriCell™ cryopreservation services provide comprehensive global support, ensuring the preservation and delivery of high-quality manufacture-ready cryopreserved leukopaks. With cryopreservation facilities strategically located in the U.S. and Europe, we offer geographic coverage that supports worldwide reach. This enables us to maintain the integrity and viability of critical materials while minimizing transportation times.
Our global capabilities are a cornerstone of IntegriCell™ services, supported by our network of facilities around the world. This extensive network allows for the efficient management of critical materials, ensuring they are preserved in optimal condition. By providing reliable and secure cryopreservation solutions, we enable the seamless integration of our services into global clinical trials, thereby enhancing the development and delivery of cutting-edge cell and gene therapies.
End-to-End Supply Chain Provider
Partnering with Cryoport Systems provides comprehensive access to our integrated, temperature-controlled supply chain solutions to optimize costs, improve efficiencies, and mitigate risks. Our comprehensive approach ensures that all aspects of the cell therapy development process, from initial cryopreservation to the final drug product, are managed seamlessly through a single vendor relationship. This integrated service model provides advanced therapy developers with a streamlined experience, allowing them to focus on their core objectives while we handle the intricacies of the sensitive temperature-controlled supply chain.
Our end-to-end services encompass cryopreservation, BioServices and biostorage, shipping systems and logistics, and customized consulting for proactive risk mitigation, all managed under one contract. This integrated framework simplifies the process for biotech teams and Contract Development and Manufacturing Organizations (CDMOs), enabling them to concentrate on their primary business functions without the added complexity of coordinating multiple vendors. By consolidating these critical services, Cryoport Systems ensures a cohesive and efficient supply chain that supports the successful development and delivery of cell and gene therapies.
Minimizing touch points in the supply chain is a key strategy for reducing risk, and Cryoport Systems excels in this area by offering combined cryopreservation, logistics, and biostorage services. This integration not only enhances operational efficiency but also significantly mitigates potential risks associated with handling and transporting sensitive biological materials. By choosing Cryoport Systems as your end-to-end supply chain provider, you gain a reliable partner dedicated to maintaining the highest standards of quality and consistency throughout the entire process.
Apheresis Sourcing & Collection Partnerships
Through strategic partnerships, Cryoport Systems significantly enhances its supply chain capabilities to provide access to highly characterized, quality-controlled, manufacture-ready cryopreserved leukopaks. By collaborating with world-renowned organizations, we enable donor material collection and management that meets the highest standards. These partnerships enable us to offer therapy developers a reliable and consistent supply of top-tier leukapheresis starting material.
Our alliances include partnerships with NMDP BioTherapies and other prominent organizations, granting us access to a pool of over 7 million genetically diverse potential donors. Our established network guarantees compliance with regulatory standards and maintains the integrity of the donor material throughout the collection process.
Cryoport Systems supports the delivery of optimized, manufacture-ready cryopreserved leukopaks across the U.S. and Europe. Our global reach and strategic partnerships enable us to meet the demands of clinical trials and commercial manufacturing processes. By leveraging these collaborations, we ensure that our clients have access to the best possible starting material, supporting the advancement of cell and gene therapies worldwide.
Empowering Advanced Cell Therapy Development and Manufacturing with IntegriCell™ Cryopreservation Services from Cryoport Systems
Explore our comprehensive capabilities for optimized, temperature-controlled supply chain support

Poster Download
Cryo-processed Leukapheresis Using Automated Closed System: A High-Quality Starting Material for Autologous and Allogeneic CAR-T Cell Therapy Manufacturing
IntegriCell™ yields high-quality starting material suitable for both autologous and allogeneic CAR-T cell therapy manufacturing.

Webinar On Demand
Roundtable: Preserving the future of medicine: standardizing leukapheresis materials with cryopreservation for scalable cell therapies
Watch this roundtable webinar for actionable insights from experienced professionals in cell therapy development, cryopreservation innovation, and supply chain optimization.

White Paper
IntegriCell™ Standardizing Cryopreservation for a Sustainable Cell Therapy Supply Chain
This white paper addresses the challenges associated with fresh materials and provides an innovative approach to high-quality, consistent cryopreserved leukapheresis used as starting materials for many cell-based therapies.
Webinar On Demand
When does outsourcing autologous collection center qualification and the cell therapy supply chain make sense?
Discover practical strategies for optimizing autologous cell therapy logistics and deciding when outsourcing collection center qualification truly makes sense for your organization’s efficiency, quality, and scalability goals.

Poster Download
Optimizing Cell Therapy with IntegriCell™ Automated Cryopreservation
Learn how IntegriCell™ optimizes cryopreservation with automated closed processing, maintaining cell viability and recovery across multiple donor-derived leukaphereses.

Video
Development of an Automated Cryopreservation Process for Leukapheresis
Dive into the details of our GMP-compatible automated cryopreservation service and discover how Cryoport Systems is enhancing the cell therapy supply chain for clinical and commercial partners globally.

Flyer
Your Comprehensive Life Science Supply Chain Partner
With our expansive platform of management solutions and decades of temperature-controlled supply chain expertise, Cryoport Systems is supporting certainty in the supply chain—one patient, one therapy, one product at a time.