Quality
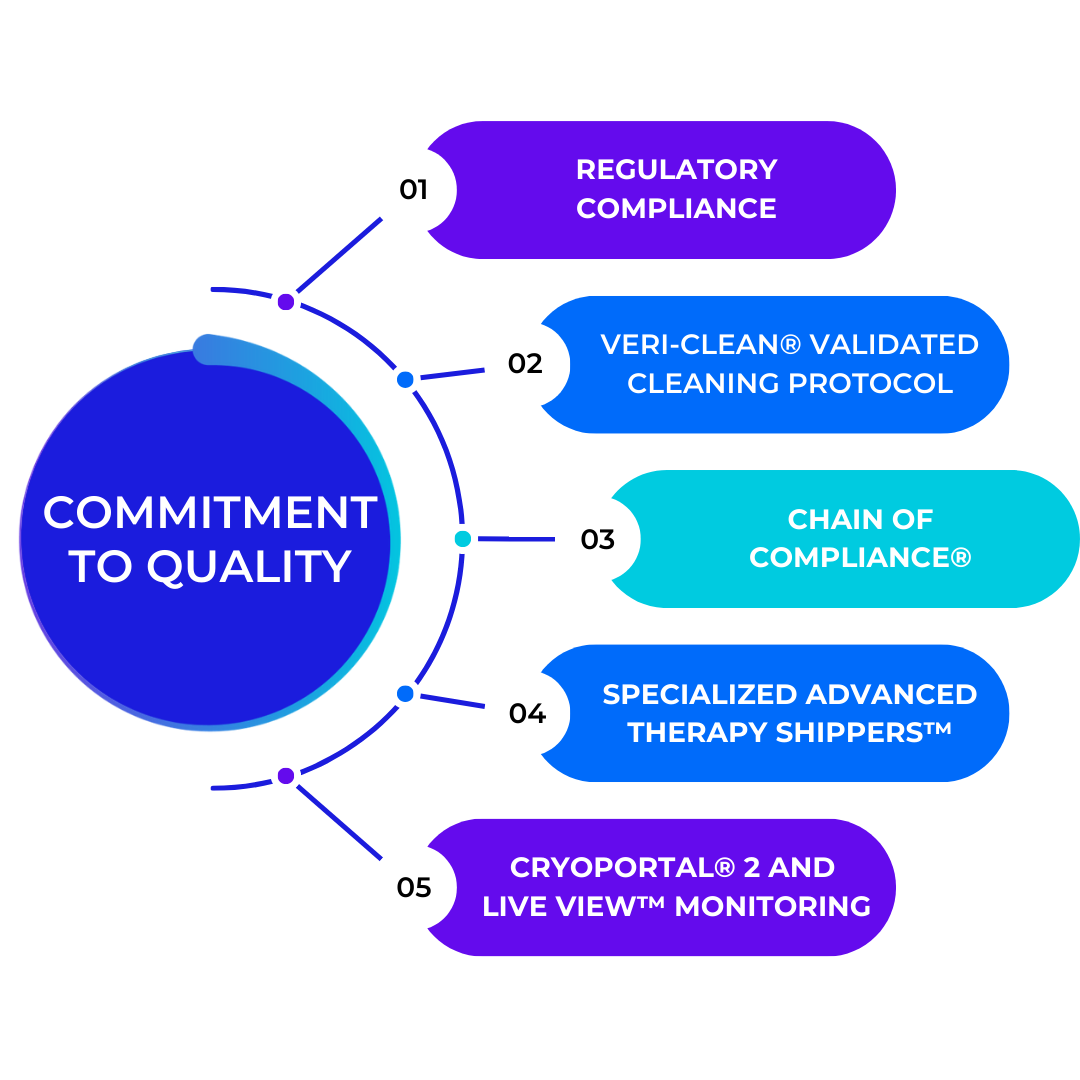
Cryoport Systems Leads the Way
In temperature-controlled supply chain solutions that mitigate risk at every turn
At Cryoport Systems, we are dedicated to setting the industry standard for quality and safety in the temperature-controlled supply chain. Our comprehensive approach to risk mitigation ensures the highest level of reliability across our entire platform of supply chain solutions.
- Industry Standardization: As pioneers in creating and adhering to ISO 21973, Cryoport Systems has played a crucial role in shaping the global standards for the safe and effective transport of temperature-sensitive materials.
- Advanced, Risk-Mitigating Protocols: With our proprietary Veri-Clean® system, all shipping containers are decontaminated to the highest standards. Additionally, we are the only provider to requalify every shipping system, every time.
- End-to-End Compliance: Our Chain of Compliance® is a comprehensive system that guarantees adherence to the highest quality standards, a critical component in meeting ISO 21973 standards for our clients.
- Innovative Monitoring Solutions: We offer state-of-the-art near real-time monitoring and data retention capabilities through our validated Cryoportal® 2 platform for regulatory compliance.
Cryoport Systems sets the standard for quality and safety in the temperature-controlled supply chain
The Cryoport Systems Quality Commitment
Unmatched Excellence in Temperature-Controlled Logistics
Cryoport Systems is committed to excellence in every aspect of our operations within our comprehensive platform of solutions. Our approach ensures the highest quality standards and reliability across the entire supply chain, with a commitment to quality that is built on a foundation of rigorous standards and innovative solutions. From our precision-engineered shipping systems that offer meticulous attention to detail for the safe transport of sensitive materials to our transportation and logistics solutions that are designed to proactively mitigate risk, we are dedicated to ensuring the integrity of your temperature-controlled supply chain at every stage.
ISO 21973: Shaping Industry Standards
Our experts were instrumental in developing ISO 21973, a standard that ensures the safe and effective transport of cells for therapeutic use. This involvement underscores our commitment to industry leadership and excellence.
- Expertise in Standardization: Our team’s contribution to ISO 21973 highlights our dedication to setting industry benchmarks and reflects our deep understanding of the complexities involved in transporting temperature-sensitive materials for the life sciences.
- Global Impact: ISO 21973 is recognized worldwide, reflecting our commitment to quality and safety on a global scale, ensuring that no matter where our clients operate, they can rely on our adherence to these stringent standards.
- Continuous Improvement: We are committed to continually improving our processes to meet and exceed ISO 21973 standards, with a proactive approach to maintaining the highest quality in all our operations.
By adhering to ISO 21973, we not only ensure compliance but also foster trust and confidence among our clients. This standardization translates to consistent, reliable services that our clients can depend on, knowing their materials are handled with the utmost care and precision.
Veri-Clean®: Advanced Cleaning Protocols
Veri-Clean® is our proprietary cleaning process that ensures the highest level of decontamination for all our shipping systems and stainless steel accessories. This process is critical for maintaining the integrity and safety of temperature-sensitive materials. Every shipping system undergoes the Veri-Clean® process every time it is returned, ensuring that all of our shipping systems are provided in a superior, decontaminated state.
- Comprehensive Decontamination: Veri-Clean® utilizes advanced techniques to achieve superior cleanliness, removing potential contaminants that could compromise the safety and efficacy of sensitive biological materials.
- Reliability and Safety: Ensures that all shipping systems are free from contaminants, providing peace of mind to our clients by safeguarding their valuable shipments against contamination risks.
- Regulatory Compliance: Meets and exceeds industry regulatory standards for decontamination, demonstrating our commitment to adhering to and surpassing the highest quality and safety protocols.
Veri-Clean® represents our dedication to maintaining a sterile and safe environment for all transported materials. This rigorous cleaning protocol not only protects the integrity of the shipments but also underscores our commitment to quality and client satisfaction.
Chain of Compliance®: Ensuring Integrity
Our Chain of Compliance® guarantees that every step of the shipping process adheres to the highest quality standards. This system ensures that all regulatory requirements are met (or exceeded) and that the integrity of the shipment is maintained throughout the entire journey. By leveraging extensive data collection and management capabilities, we mitigate risks and implement preventative measures to ensure the safe delivery of invaluable materials.
- End-to-End Traceability: Every shipment is tracked and documented to ensure compliance with all regulatory requirements, providing a clear and auditable trail that reinforces the reliability of our services.
- Data-Driven Risk Mitigation: Our comprehensive approach minimizes risks and ensures the safe delivery of temperature-sensitive materials, reducing potential disruptions and ensuring the highest level of care and precision.
- Validated Requalification Procedures: We are the only provider to requalify every shipping system, every time to ensure that all equipment continues to meet rigorous standards before each use.
Our Chain of Compliance® integrates validated requalification procedures, segregates human- and animal-derived products, and maintains a complete history of equipment performance and location. Our clients can be confident that their shipments are managed with the highest standards of care and compliance at every step.
Advanced Therapy Shippers (ATS): Specialized Solutions
Our Advanced Therapy Shippers (ATS) are exclusively designed for the transportation of engineered human cell and gene therapies and human-derived cellular and biological materials. Our ATS fleet is segregated from our General Purpose (GP) fleet and developed to meet the highest standards of validation and decontamination, providing unmatched confidence in the safety and integrity of your critical therapies.
- Exclusive Use: ATS shipping systems are dedicated solely to human cell and gene therapies, segregated from GP shipping systems to prevent any possibility of cross-contamination.
- Meets or Exceeds Compliance Regulations: By anticipating and addressing future regulatory requirements within the temperature-controlled supply chain, we ensure that our shipping systems and services remain at the forefront of innovation and compliance.
- Certificate of Conformance: For an additional layer of risk mitigation and protection, our Certificate of Conformance is a formal certification, signed by a quality assurance (QA) team member, that certifies that every unique shipping system has only handled human cell and gene therapy products.
By partnering with Cryoport Systems, you are ensuring that your critical cell and gene therapies are transported safely, reliably, and in compliance with the highest industry standards.
Cryoportal® 2: Near Real-Time Monitoring
Cryoportal® 2 is our state-of-the-art logistics management platform that offers near real-time monitoring and data retention. This platform provides clients with complete visibility into the status of their shipments, ensuring transparency and accountability. Our Cryoportal® 2 is validated to demonstrate compliance with Code of Federal Regulations 21 CFR Part 11 and the International Society for Pharmaceutical Regulations Good Automated Manufacturing Practices (ISPE GAMP).
- Enhanced Visibility: Provides ongoing updates on shipment status, location, and condition, allowing clients to monitor their shipments closely and respond swiftly to any issues that may arise.
- Data Retention: Maintains detailed records of all shipments for compliance and auditing purposes, ensuring that all historical data is readily accessible for regulatory and quality assurance needs.
- Client Access: Offers clients 24/7 access to shipment information, enhancing communication and trust by providing a seamless and transparent view into the logistics process.
Cryoportal® 2 empowers our clients with the information they need to make informed decisions and maintain oversight of their critical shipments. This advanced platform reflects our commitment to leveraging technology to enhance service quality and reliability.
Transforming industry standards into exceptional performance

Download the ISO 21973 Whitepaper
The cell and gene therapy industry requires end-to-end precision and traceability — everything from chain of custody to chain of condition and chain of identity. ISO 21973:20202 extends the chain …

Chain of Compliance
Our Chain of Compliance® guarantees that every step of the shipping process adheres to the highest quality standards. This system ensures that all regulatory requirements are met (or exceeded)…

Download the Case Study
When Cryoport’s client experienced a problem with shippers tilting during transit, compromising the effectiveness of the liquid nitrogen coolant and jeopardizing a CAR T-cell immunotherapy clinical program…

Veri-Clean®
Veri-Clean® is our proprietary cleaning process that ensures the highest level of decontamination for all our shipping systems and stainless steel accessories. This process is critical for maintaining the integrity and safety…

Chain of Compliance White Paper
As companies increasingly add regenerative medicine therapies to their pipelines, they must examine their supply chain. Not only are these fragile materials often irreplaceable, but they are also more at risk…

Complying with Critical Regulations
With Cryoport’s unique Chain of Compliance, we are able to meeting all critical regulations regarding the history, quality, and processing of our cryoshippers during all stages of shipment. By providing…

Download the Case Study
Smartpak II™ Alert and Prompt Intervention Saves the Day. When a liquid nitrogen shipper was placed on its side in transit, potentially compromising the cryogenic temperature, Cryoport’s customer service team…
Advanced Therapy Shippers®
These unique cryo-shippers are created and certified for the sole purpose of transporting human clinical and commercial drug products. Through our Veri-Clean™ disinfecting procedures…
At Cryoport Systems, quality is not just a goal, it’s a guarantee
Quality you can TRUST
At Cryoport Systems, we are purpose-built for robust, comprehensive solutions with a longstanding dedication to quality.
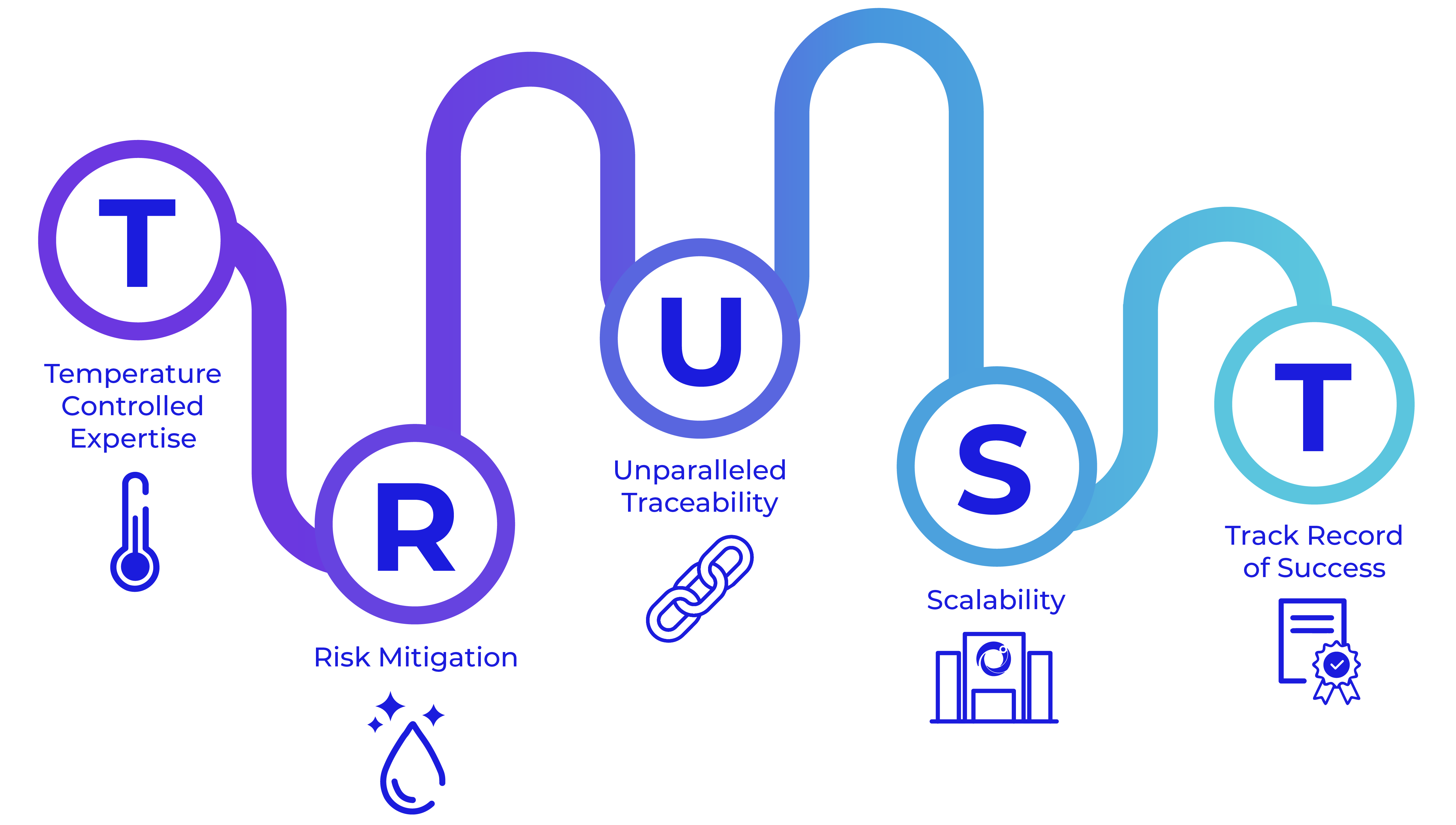
We lead the way in temperature-controlled expertise for supply chain support with agile, flexible solutions that meet your needs. Our consistent risk mitigation, from our proprietary Veri-Clean® process to our robust requalification procedures, means we are the only provider to requalify our shippers – every shipment, every time. We stand at the forefront with unparalleled traceability, thanks to our Chain of Compliance® for full traceability in managing the distribution of irreplaceable commodities. We offer scalability with an expanded depth of services – such as BioServices and biostorage, consulting, and cryopreservation – that add certainty to your supply chain with tailored solutions that fit your needs. With 650+ clinical trials supported and more than half a million successful shipments of sensitive – and often irreplacable – materials across the globe, you can count on our track record of success that spans decades.