Support for Phase III Clinical Programs

Phase III Clinical Trial Support that’s Scalable, Compliant, and Ready for Commercialization
Partner with Cryoport Systems to streamline complexity and safeguard your pivotal trials with an end-to-end supply chain platform built for scalability
Phase III trials represent the most demanding stage of clinical development, where operational complexity and regulatory scrutiny reach their peak. Sponsors face the challenge of scaling patient populations, onboarding multiple global sites, and maintaining absolute consistency and compliance across every process. At this stage, fragmented supply chains and vendor transitions can introduce costly delays and compliance risks that jeopardize timelines and BLA approval. Cryoport Systems eliminates these risks through a single-vendor partnership that integrates every critical component of your supply chain under one cohesive platform.
Our solutions are designed to anticipate downstream needs, ensuring your Phase III operations transition seamlessly into commercialization without rework or disruption. With standardized processes across our global network and ISO 21973-certified workflows, we deliver reproducibility and audit-ready documentation with risk mitigation at scale. By partnering with Cryoport Systems, you gain a strategic ally committed to protecting patient safety and accelerating timelines, securing your commercial runway.
Full End-to-End Support
A unified platform for every phase of your supply chain
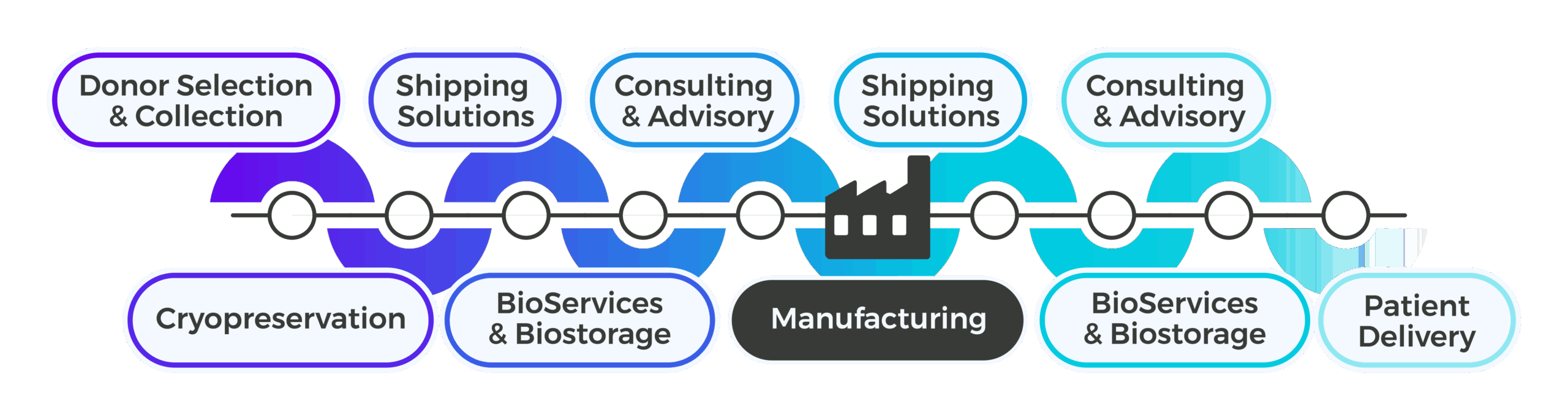
Support Upstream of Manufacturing
Streamline early-phase complexity to protect downstream scalability
Phase III success begins with upstream precision. Sponsors often underestimate the impact of early-stage variability on large-scale trials, leading to inefficiencies that ripple through manufacturing and distribution. Cryoport Systems mitigates these risks by embedding standardization and compliance into upstream processes, including kit design, cryopreservation strategies, and biostorage planning. Our experts collaborate with your team to ensure alignment across all sites, reducing operational burden and eliminating the need for knowledge transfers later. By leveraging our integrated platform, you gain continuity from collection to manufacturing, supported by validated workflows and risk-based planning. This proactive approach safeguards timelines and positions your program for seamless progression into commercialization.
- Custom kit design and production for consistent sample collection
- Integrated biostorage solutions for GMP readiness
- Cryopreservation strategies to maintain material integrity
- Risk-based logistics planning to anticipate downstream needs
Upstream precision is the foundation for Phase III scalability. By partnering with Cryoport Systems early, you eliminate variability that can compromise trial integrity and regulatory confidence. Our solutions reduce complexity and mitigate risk, creating a unified framework that supports every milestone. This level of control ensures that your pivotal trials progress without disruption and your commercial runway remains clear. With Cryoport Systems, upstream planning becomes a catalyst for success.
Support Downstream of Manufacturing
Accelerate timelines with standardized processes and global reach
As Phase III trials expand, downstream operations become increasingly complex. Sponsors must manage large-scale distribution, maintain compliance across multiple geographies, and prepare for commercial launch, all without sacrificing precision. Cryoport Systems delivers continuity through a single-vendor platform that integrates logistics, biostorage, and secondary packaging and labeling under one roof. Our global network ensures proximity to patient populations, reducing transit times and mitigating risk. With standardized SOPs and ISO-certified workflows across all facilities, we operate with industry-leading reproducibility and audit-ready documentation at scale. Our experts provide personalized consultation to align downstream processes with regulatory expectations, ensuring readiness for BLA submission and commercialization.
- Global logistics network for efficient trial coverage
- ISO 21973-certified workflows for compliance assurance
- Chain of Compliance® for full traceability and audit readiness
- Rapid local delivery through Cryoshuttle® service in select locations
- Integrated BioServices and biostorage for continuity
- Qualified Person (QP) and regulatory support for release into the EU
Downstream operations are where timelines are won or lost. Cryoport Systems provides the infrastructure and expertise to keep your program moving forward without compromise. Our solutions reduce variability and safeguard patient materials while ensuring compliance across every touchpoint. By partnering with us, you gain a strategic advantage that accelerates progress and secures your path to commercialization. With Cryoport Systems, downstream complexity becomes a controlled, predictable process.
Cryopreservation for Consistency and Scale
Standardized solutions to protect integrity across global trials
Cryopreservation of starting material is critical to maintaining material integrity during Phase III trials, where variability can compromise patient safety and regulatory confidence. Cryoport Systems delivers standardized cryopreservation solutions through our IntegriCell® platform, designed to ensure consistency across all sites and phases. Our automated closed processes reduce contamination risk and maintain reproducibility, enabling seamless scale-up for large patient populations. By implementing these solutions early, sponsors avoid the disruptions that come from fragmented cryopreservation strategies. Our experts provide guidance on best practices and compliance alignment to protect your materials throughout the supply chain, extending manufacturability of critical starting materials while maintaining cellular viability. Combined with integrated biostorage and validated logistics, Cryoport Systems creates a unified framework that safeguards integrity from collection to commercialization.
- Automated closed cryopreservation processes for consistency
- Integrated biostorage for GMP compliance
- Risk-based monitoring and documentation for audit readiness
- Scalable solutions to support global trial expansion
Cryopreservation of leukapheresis-derived starting materials is a strategic differentiator for Phase III success. Cryoport Systems ensures that every sample is preserved with precision, reducing variability and protecting patient safety while extending manufacturability and offering integrated biostorage for just-in-time delivery of cryopreserved materials to manufacturing. Our standardized solutions create continuity across sites and phases, enabling scalability without compromise. By partnering with us, you gain confidence that your materials will maintain integrity throughout the trial lifecycle.
BioServices for Operational Efficiency
Simplify complexity with integrated BioServices and biostorage
Phase III trials demand operational efficiency at scale, and fragmented BioServices can introduce delays and compliance risks. Cryoport Systems eliminates these challenges through integrated solutions that combine kit production, secondary packaging and labeling, clinical sample management, biostorage, and logistics under one platform. Our experts design and produce custom collection and manufacturing kits tailored to your trial requirements, ensuring consistency across all sites. By managing these processes within a single-vendor relationship, we reduce operational burden and eliminate the need for multiple handoffs. Our GMP-aligned biostorage facilities provide secure, compliant housing for sensitive materials, supported by continuous monitoring and audit-ready documentation. Our Qualified Person (QP) and regulatory support enables global expansion and release of drug product into the EU. This level of integration streamlines workflows, accelerates timelines, and positions your program for seamless progression into commercialization.
- Custom kit design and production for trial-specific needs
- GMP-compliant biostorage solutions for material integrity
- Qualified Person (QP) release for European expansion
- Integrated logistics for end-to-end continuity
- Continuous monitoring and documentation for compliance assurance
- Risk-based planning to anticipate future scalability
BioServices are the backbone of operational efficiency during Phase III trials. Cryoport Systems delivers these services within a unified platform, reducing complexity and safeguarding compliance. Our solutions create continuity across every touchpoint, enabling sponsors to focus on science rather than supply chain management. By partnering with us, you gain a strategic advantage that accelerates progress and protects patient safety, transforming BioServices into a driver of success.
Consulting and Advisory Support for Strategic Alignment
Expert guidance to navigate complexity and ensure compliance
Phase III trials introduce regulatory and operational challenges that require expert guidance. Cryoport Systems provides consulting and advisory services designed to align your supply chain strategy with global compliance standards and commercial readiness. Our experts collaborate with your team to develop risk mitigation plans and continuity strategies that anticipate future needs. By embedding these practices early, sponsors avoid costly rework and maintain momentum toward BLA approval. Our advisory services extend beyond logistics, offering insights into regulatory requirements, import/export compliance, and best practices for global scalability. This proactive approach ensures that your supply chain remains resilient, compliant, and ready for commercialization.
- Risk-based lane qualification for international and complex routes
- Packaging validation aligned with global regulatory standards
- Commercial runway planning and compliance readiness
- Strategic insight from thousands of advanced therapy programs
Consulting and advisory services are essential for navigating the complexity of Phase III trials. Cryoport Systems provides the expertise and foresight to keep your program on track and compliant. Our solutions reduce uncertainty, mitigate risk, and create a clear path to commercialization. By partnering with us, you gain confidence that every decision supports long-term success.
Shipping Systems for Reliability and Control
Validated solutions to protect patient materials across every mile
Phase III trials require shipping systems that deliver reliability at scale. Cryoport Systems provides fully validated solutions designed to maintain material integrity across every journey. Our diverse fleet supports all temperature bands, including cryogenic, ultra-cold, and controlled room temperature, ensuring compatibility with any therapy type. Each system is equipped with Smartpak™ condition monitoring technology for continuous monitoring and integrated with our Cryoportal® logistics management platform for full visibility. This proactive approach enables rapid intervention if deviations occur, safeguarding patient materials and trial integrity. Combined with our Veri-Clean® decontamination and full requalification process of every shipping system before every use, as well as full Chain of Compliance® documentation, Cryoport Systems delivers unmatched control and audit-ready transparency. Our solutions reduce variability, mitigate risk, and ensure that every shipment meets the highest standards of quality and compliance.
- Diverse fleet for all temperature bands and therapy types
- Smartpak™ condition monitoring technology for continuous monitoring
- Cryoportal® logistics management platform for visibility and reporting
- Veri-Clean® decontamination and requalification for system integrity
- Chain of Compliance® for full traceability and audit readiness
Shipping systems are the lifeline of Phase III trials, and reliability is non-negotiable. Cryoport Systems delivers solutions that combine technology, compliance, and scalability to protect patient materials across every mile. Our integrated platform reduces operational burden and provides the transparency needed for regulatory confidence. By partnering with us, you gain control over one of the most critical aspects of your trial.
Quality & Compliance for Risk Mitigation
Proactive strategies to safeguard timelines and regulatory confidence
Phase III trials operate under intense regulatory scrutiny, and compliance gaps can derail progress. Cryoport Systems mitigates these risks through a culture of quality and compliance embedded into every process. Our ISO 21973-certified workflows, Chain of Compliance® documentation, and continuous monitoring technology ensure that every shipment meets stringent standards. We provide audit-ready reports and risk-based planning to anticipate and prevent disruptions before they occur. Our experts collaborate with your team to develop strategies that align with GMP and global regulatory requirements. This proactive approach reduces uncertainty, safeguards timelines, and positions your program for successful commercialization. With Cryoport Systems, compliance becomes a source of confidence rather than concern.
- ISO 21973-certified workflows for global compliance
- Chain of Compliance® for full traceability and audit-readiness
- Continuous monitoring technology for proactive risk mitigation
- Risk-based planning to anticipate and prevent disruptions
Quality and compliance are the cornerstones of Phase III success. Cryoport Systems delivers solutions that protect patient safety and maintain regulatory confidence, ultimately accelerating progress toward commercialization. Our proactive strategies reduce risk and create a clear path forward, even in the face of complexity. By partnering with Cryoport Systems, you gain assurance that every process meets the highest standards.
Featured Resources

Article
Beyond the Box: Why Shipping System Qualification Matters in Advanced Therapies
For advanced therapy logistics, quality isn’t limited to what happens inside the manufacturing suite. It extends across the entire supply chain, from packaging and transport to the systems that safeguard material integrity at every handoff. Yet one of the most critical (and often underestimated) steps…
Case Study
When Every Hour Counts: How Cryoport Systems Rapidly Mobilized to Protect INmune Bio’s Critical Clinical Materials
For INmune Bio Inc., a clinical-stage biotechnology company developing novel biotherapeutics, that infrastructure was put to the ultimate test when a sudden biostorage site closure threatened to derail a pivotal clinical trial…

Article
Scale Smarter: How Global Supply Chain Centers Amplify CGT Program Growth
Cell and gene therapy (CGT) programs are among the most complex and sensitive operations in the life sciences. From cryopreservation and biostorage to logistics and regulatory compliance, every step in the supply chain can influence product integrity, clinical timelines, and…
Jumpstart your Phase III Clinical supply chain with Cryoport Systems and build a strategy that’s scalable, fully compliant, and ready for every phase ahead.
- IntegriCell® Cryopreservation
Consistent, high-quality leukapheresis starting material.
Learn More >> - BioServices & Biostorage
Integrated solutions to support advanced therapies.
Learn More >> - Consulting & Advisory Services
Our team of experts can help de-risk your logistics.
Learn More >> - Shipping Solutions
Temperature-controlled packaging solutions.
Learn More >> - Transportation
Transportation of commodities at various temperatures.
Learn More >>
Cryoport Systems is Enabling the Outcome™ through our unparalleled supply chain platform.
Offering a comprehensive scope of robust supply chain management solutions and trusted logistics.
Cryoport Systems was established by a team of doctors dedicated to serving the life sciences through expert logistics support. Today, our partnership capabilities have evolved beyond logistics into a robust platform of supply chain solutions that supports every stage and phase of the life sciences research, development, and manufacturing process. Our comprehensive platform of solutions makes Cryoport Systems a standalone vendor that can support all aspects of the temperature-controlled supply chain.
LEARN MORE ABOUT HOW CRYOPORT SYSTEMS IS ENABLING THE OUTCOME™






