Get in touch with our team of experts:
Global Experts in Temperature-Controlled Supply Chain Management for Critical Materials.
Cryoport Systems is your strategic partner for comprehensive solutions that protect high-value biological materials throughout the supply chain.
Our patented, state-of-the-art shipping systems, integrated global BioServices and biostorage capabilities, continuous cloud-based monitoring, and efficient local pickup and delivery services combined with tailored consulting services maximize the safety and quality of your life-saving therapies and critical materials.
- Risk Mitigation: We are committed to your success
From comprehensive traceability to our Veri-Clean® process, we are the only provider to requalify our shippers – every shipment, every time. - Trust: Lean on our decades of experience
We’ve supported 650+ clinical trials and have played a significant role in the commercialization of over a dozen products. - Agility: Flexible solutions to meet your needs
Our BioServices and biostorage solutions expand our flexible and scalable biomaterials management capabilities to every aspect of the supply chain for the discovery, development, and delivery of cell and gene therapies. - Scalability: Comprehensive services for certainty in your supply chain
And we’re just getting started! Our continued growth and evolution of services add certainty to your supply chain with tailored solutions that fit your needs.
Enabling the Outcome™ for the Biopharmaceutical, Animal Health, and Reproductive Medicine industries.
At Cryoport Systems, our people, innovative solutions, and leading technologies go above and beyond to help deliver certainty throughout the life sciences supply chain.
Our robust quality standards ensure companies meet all critical supply chain regulations, and our teams of specialists, alongside our innovative platform of industry-leading solutions, enable our clients’ journeys from initial research to global commercialization. We offer comprehensive solutions specifically designed for various biopharmaceutical, animal health, and reproductive medicine applications.
Biopharma
Our robust supply chain management solutions and trusted logistics are Enabling the Outcome™ of groundbreaking discoveries and life-saving therapies, impacting patients around the world.
With solutions poised to sustain the various critical stages and phases throughout biopharmaceutical research, development, and manufacturing, Cryoport Systems is the global leader in temperature-controlled supply chain expertise. Our BioServices and biostorage offerings and IntegriCell™ cryopreservation services, combined with our expert logistics capabilities, actively mitigates risk, making Cryoport Systems the single vendor that can support all aspects of the biopharmaceutical, temperature-controlled supply chain while actively ensuring our clients reach their defined outcomes.
Animal Health
Our robust supply chain management solutions and trusted logistics are Enabling the Outcome™ of our clients’ groundbreaking therapy research, critical vaccine development, and elite genetics management for the animal health industry.
Our platform of solutions are poised to sustain the stages and phases throughout the animal health research, development, and transport journeys. With our risk-mitigating services like Veri-Clean®, the industry’s first and only validated shipper cleaning and disinfection procedure, coupled with our expert logistics services that actively mitigate risk through our Chain of Compliance®, Cryoport Systems is the single vendor that can support all aspects of the temperature-controlled supply chain while enabling our clients to achieve their defined outcomes in the animal health market.
Reproductive Medicine
Our robust and trusted logistics are Enabling the Outcome™ for intended parents on their fertility journeys and for the clinics that care for their clients’ reproductive health.
Our platform encompasses solutions to assist the transport and storage needs of clinics and intended parents throughout their fertility journeys. With our risk-mitigating services like Veri-Clean®, the industry’s first and only validated shipper cleaning and disinfecting procedure, coupled with our expert logistics services that actively mitigate risk through our Chain of Compliance®, Cryoport Systems is the single vendor that can support all aspects of the temperature-controlled supply chain while enabling our reproductive medicine clients toward their defined outcomes.
Supporting certainty through our risk-mitigating supply chain expertise — one patient, one therapy, one product at a time.
Download the ISO 21973 Whitepaper

This white paper discusses key recommendations and changes that interested parties should review and evaluate when planning their clinical and commercial distribution strategies for cell and gene therapies.
Global Compliance, Local Presence
We have created a platform of temperature-controlled supply chain solutions that consistently meet or exceed industry standards.
At Cryoport Systems, we are driving standardization to address the critical complexities related to the collection and processing of patient material, mitigating risk at every stage. Our experts have contributed to developing ISO standards, such as ISO 21973 (General requirements for transportation of cells for therapeutic use), to help drive best practices in the industry. We work closely with ISTA, IATA, ASTM, and other internationally accredited organizations. We are also diligently involved with the Foundation for the Accreditation of Cellular Therapy (FACT) and the Standards Coordinating Body (SCB).
Our operating procedures are uniform across all of our facilities, ensuring consistent support across all stages and phases of your journey regardless of which global Cryoport Systems location you’re working with.
We stand at the forefront of complete traceability in the supply chain, with complete temperature records of transported materials maintained for at least 10 years and an extensive historical data depository to help guide risk-based decision-making. Our Cryoportal® Logistics Management System is the industry’s only system with validated compliance to 21 CFR Part 11 and ISPE GAMP5.
Unparalleled Scope of Robust Supply Chain Management Solutions and Trusted Logistics
Cryoport Systems is Enabling the Outcome™ through our unparalleled supply chain platform.
As the life sciences continue to evolve and advance, so do we. Our integrated supply chain services are in a league of their own, safely delivering products, therapies, and treatments that require specialized temperature-controlled management. Our comprehensive platform of solutions makes Cryoport Systems a standalone vendor that can support all aspects of the temperature-controlled supply chain.
IntegriCell™ Cryopreservation
Unmatched in our temperature-controlled supply chain expertise, Cryoport Systems has integrated cryopreservation services into our platform of solutions for cell and gene therapy developers that require the collection, cryopreservation, distribution, and storage of advanced therapies.
Our standardized cryopreservation protocols and use of innovative technologies support the biopharmaceutical supply chain from research stages to clinical operations and process development to manufacturing. Integrated into a platform of temperature-controlled logistics excellence, storage and distribution capabilities, and industry-leading cryopreservation services, IntegriCell™ helps to ensure the safety, quality, and viability of critical biotherapeutic materials.
Shipping Systems
Cryoport Systems has developed and implemented the only premier temperature-controlled shipping systems in the industry that adhere to ISO 21973 standards and support various temperature ranges. While other companies rely on rented dewars to complete shipments, Cryoport Systems created diverse shipper systems referred to as Cryoport Elite® and Cryoport Express®.
Our shipping systems undergo meticulous engineering to achieve unparalleled quality. They’re also supported by Veri-Clean®, our fully validated cleaning and disinfection process, along with our Chain of Compliance®, which provides end-to-end accountability. We incorporate state-of-the-art informatics and data monitoring technology into our systems to provide near-real time traceability. Clients trust us because our processes are proven to maintain both Good Manufacturing Practice (GMP) and Good Distribution Practice (GDP) standards no matter the material type.
BioServices & Biostorage
We’re proud to introduce our BioServices solutions at our Global Supply Chain Centers. These state-of-the-art facilities combine our existing logistics processes and capabilities with our new, cutting-edge Bioservices infrastructure all under one roof.
We’ve designed this new and integrated approach to support cell and gene therapy manufacturing and development. This includes comprehensive controlled-temperature storage, fulfilment, kit production, secondary packaging, labelling of therapeutic products, and storage of GMP raw materials all in addition to our world-class logistics.
Consulting Services
Complex biologics and cell & gene therapies need a robust, temperature-sensitive supply chain. These chains are often more complex than a normal supply chain. With our consulting services, you have an expert at hand who is full of crucial advice and knowledge and who can quickly spot and solve any risks in your temperature-controlled supply chain and logistics. We tailor solutions — such as shipping risk assessments, shipping lane qualifications and shipper/packaging qualifications — that deliver value and insight, with robust solutions that are customizable to your stage of development. Whether you are pre-IND or post-commercial, we work with you to tailor a solution that meets your needs, timelines, and budget.
Transportation
When it comes to shipping sensitive materials, Cryoport Systems’ transportation services can be trusted every step of the way. Our logistics capabilities meet the transportation needs of a wide range of materials for the biopharmaceutical, reproductive medicine, and animal health markets. We proudly provide the safe transportation of commodities at temperatures including cryogenic, ultra-cold (dry ice), 2-8˚C, and controlled room temperature. For assistance in moving a single sample across town or shipping an entire fleet of sensitive material from one country to another, you can rely on us and our logistics partners to transport your commodities with their integrity intact.
Additionally, our Cryoshuttle® pickup and delivery service provides local sensitive material transportation within proximal distance of all Cryoport Systems Global Logistics Centers and Global Supply Chain Centers. For materials that need to be shipped internationally, our team of knowledgeable experts can help coordinate customs clearance across borders. Whether it’s domestic or global sensitive material handling, we can accommodate your temperature-controlled supply chain needs.
You can TRUST our Comprehensive and Integrated Supply Chain Platform to Enable Your Outcomes
Let’s start a conversation – tell us how we can help:
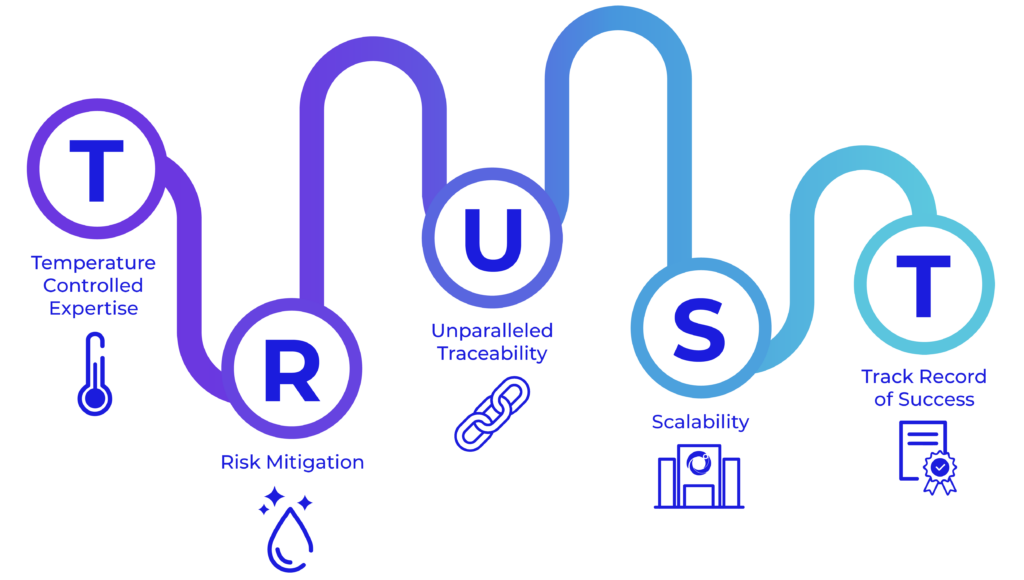
At Cryoport Systems, we are purpose-built for robust, comprehensive solutions with a longstanding dedication to quality.
We lead the way in temperature-controlled expertise for supply chain support with agile, flexible solutions that meet your needs. Our consistent risk mitigation, from our proprietary Veri-Clean® process to our robust requalification procedures, means we are the only provider to requalify our shippers – every shipment, every time. We stand at the forefront with unparalleled traceability, thanks to our Chain of Compliance® for full traceability in managing the distribution of irreplaceable commodities. We offer scalability with an expanded depth of services – such as BioServices and biostorage, consulting, and cryopreservation – that add certainty to your supply chain with tailored solutions that fit your needs. With 650+ clinical trials supported and more than half a million successful shipments of sensitive – and often irreplacable – materials across the globe, you can count on our track record of success that spans decades.