Support for Phase II Clinical Programs

Phase II Clinical Solutions for Advanced Therapy Developers
Scale with confidence by building a global-ready supply chain for expanding trials
Phase II trials represent a critical inflection point for advanced therapies. As patient populations grow and trial complexity increases, the need for a scalable and compliant supply chain that is globally integrated becomes urgent. Cryoport Systems partners with you to ensure that your Phase II program is built for continuity into Phase III and commercialization without costly revalidation or vendor switches.
Our single-vendor supply chain platform approach integrates key services like cryopreservation, BioServices and biostorage, validated logistics, and consulting and advisory services under one framework, reducing operational burden and risk. With ISO 21973-certified processes, Chain of Compliance® tracking, and integrated condition monitoring, we deliver transparency and audit-ready documentation for every shipment. From collection and manufacturing kit standardization to global distribution strategies, our solutions anticipate future demands while protecting patient safety today. By choosing to partner with Cryoport Systems, you secure a supply chain that scales seamlessly and positions your therapy for success across every milestone.
Your Partner for Global-Ready Phase II Trials
Comprehensive solutions that enable scalability and compliance
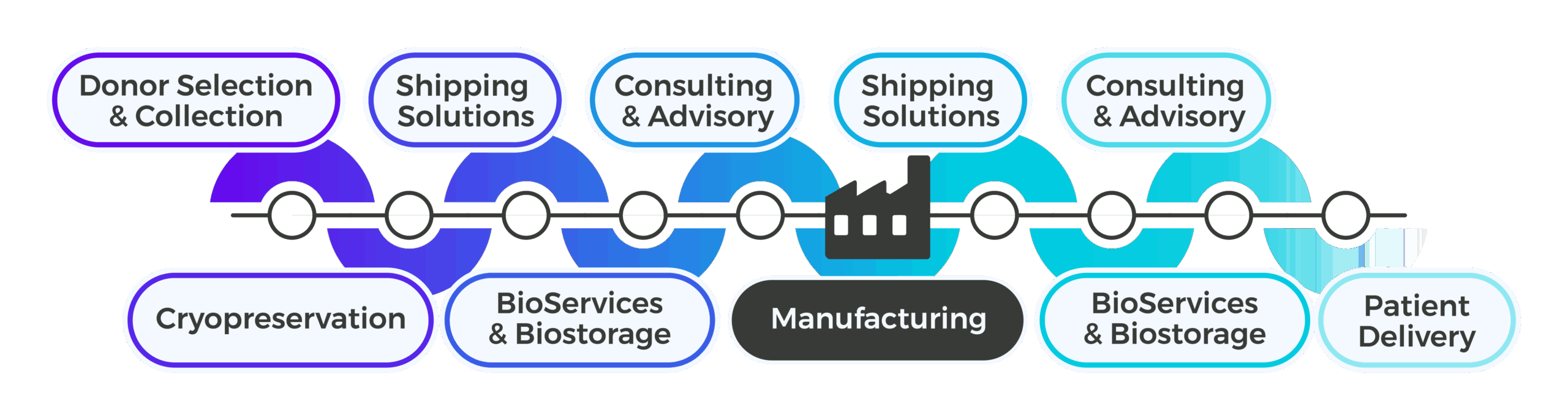
Support Upstream of Manufacturing
Plan for Scalability and Global Readiness
Phase II trials introduce larger patient populations and multi-site coordination, making upstream planning essential for reproducibility and compliance. Cryoport Systems works with your team to design GMP-aligned workflows and supply chain management strategies that anticipate Phase III requirements. Our experts help you integrate collection kit standardization, cryopreservation strategies, and biostorage planning early to reduce variability and avoid costly rework. With global facilities and uniform procedures across regions, you can scale confidently without compromising quality, making every upstream decision a competitive advantage.
- GMP and ISO 21973 compliance for scalability
- Custom, standardized collection and manufacturing kits
- End-to-end integration that incorporates cryopreservation, logistics, and biostorage
- Global facility access to support multi-site trial expansion
- Advisory services for proactive risk mitigation and regulatory filing support
Phase II is where operational complexity accelerates, and under these conditions, upstream planning plays an outsized role in determining success. Partnering with Cryoport Systems ensures your processes are standardized and scalable, designed to be audit-ready from the start. Our proactive approach reduces risk while eliminating inefficiencies, ultimately positioning your program for seamless progression into Phase III. By embedding global readiness into your workflows now, you avoid disruptions and maintain confidence as patient populations grow.
Support Downstream of Manufacturing
Deliver Materials Reliably Across Expanding Networks
As trials expand, downstream logistics become increasingly complex. Cryoport Systems provides validated shipping systems equipped with integrated condition monitoring, with full Chain of Compliance® tracking to ensure every shipment meets regulatory standards with audit-ready equipment and shipment history. Our fully owned fleet of shipping systems eliminates capacity constraints as your volume scales, while our industry-leading quality and compliance processes like Veri-Clean® decontamination and full requalification protocols before every shipment ensures shipper integrity. ISO 21973-certified processes and risk-based lane selection reduces variability and protects timelines, while Cryoshuttle® service adds rapid local pickup and delivery for first and last mile, adding another layer of protection for sensitive materials.
- Integrated Smartpak™ for continuous monitoring of every shipment throughout transit
- Chain of Compliance® enables audit-ready data for IND readiness
- Veri-Clean® decontamination and requalification of every shipping system before every use
- ISO 21973-certified workflows and processes for industry-leading compliance and quality
- Cryoshuttle® for specialized local pickup and delivery available in select locations
Phase II trials demand logistics that scale without sacrificing reliability. Cryoport Systems delivers solutions that maintain integrity across every shipment, regardless of distance or complexity. Our integrated systems provide transparency, audit-ready documentation, and proactive risk mitigation to help keep your program on track. Partnering with Cryoport Systems provides confidence that patient-ready materials will be handled to the industry’s highest standards of quality and compliance, even as trial demands grow.
Cryopreservation that Scales with Your Program
Protecting Starting Material Across Expanding Trials
Cryopreservation during Phase II is critical to maintaining consistency as patient populations grow. Cryoport Systems’ IntegriCell® solution provides validated protocols and expert guidance to ensure reproducibility across sites and batches. By implementing our Automated Closed Process early, you reduce variability and enable smooth tech transfer into GMP environments. Our IntegriCell cryopreservation services ensure compliance and scalability.
- Validated cryopreservation protocols for leukapheresis-derived starting materials
- Proprietary, validated Automated Closed Process for industry-leading consistency
- Seamless GMP integration
- Enhanced sample integrity and viability
As trials expand, variability becomes a major risk to patient safety and regulatory compliance. Cryoport Systems helps you implement cryopreservation strategies that protect material integrity and extend manufacturability while standardizing processes across clinical sites. This consistency supports reproducibility and accelerates timelines. By embedding these safeguards into Phase II, you avoid disruptions and prepare for global scale.
Integrated BioServices for Global Trials
Standardize Collection, Storage, and Management Across Sites
Phase II trials introduce complexity that demands more than basic biostorage. As patient populations grow and trial sites expand internationally, sponsors need a solution that ensures consistency and compliance across every location. Cryoport Systems delivers integrated BioServices that combine GMP-ready biostorage with global kit production for sample collection and manufacturing. Our standardized kits reduce variability and simplify onboarding for new sites, while centralized biostorage facilities maintain chain of custody and audit-ready documentation. Clinical sample management capabilities provide full visibility into every step, ensuring reproducibility and regulatory alignment. By embedding these services early, you eliminate inefficiencies and prepare for seamless progression into Phase III and commercialization.
- Global GMP-ready biostorage facilities for centralized control
- Standardized collection and manufacturing kits for reproducibility
- Clinical sample management with full traceability
- Continuous monitoring and audit-ready documentation
- Integrated logistics and cryopreservation support for scalability
Managing samples across multiple geographies during Phase II can quickly become a source of risk and inefficiency. Cryoport Systems mitigates these challenges by delivering a unified BioServices framework that standardizes processes and ensures compliance worldwide. Our approach reduces variability while accelerating site onboarding, which provides the transparency regulators expect. By consolidating biostorage and kit production under one global partner, you avoid duplication and hidden costs while maintaining operational continuity. With Cryoport Systems, BioServices become a cornerstone of scalability and a safeguard for patient safety as your program advances toward Phase III.
Strategic Consulting for Global Scale and Phase III Readiness
Navigate Complexity and Prepare for Commercial Expansion
Phase II trials are where operational complexity accelerates. Sponsors must manage larger patient populations, multiple geographies, and evolving regulatory expectations, all while preparing for Phase III and commercialization. Cryoport Systems provides consulting and advisory services that go beyond logistics planning. Our experts help you design standardized processes with fully qualified shipping lanes as routes grow more complex and implement risk-based strategies that anticipate future scale. We deliver FDA-required risk assessments, packaging validations, and compliance frameworks that eliminate surprises during audits. By embedding scalability and global readiness into your process now, you avoid costly rework and delays later.
- Risk-based lane qualification for international and complex routes
- Packaging validation aligned with global regulatory standards
- Commercial runway planning and compliance readiness
- Strategic insight from thousands of advanced therapy programs
Phase II is focused on managing today’s complexity at the same time as it’s building the foundation for tomorrow’s success. Cryoport Systems helps you anticipate challenges before they arise, ensuring your supply chain is ready for global scale and regulatory scrutiny. Our advisory services reduce operational burden and streamline decision making while providing confidence that every process meets the highest standards. By partnering with Cryoport Systems, you set yourself up with a roadmap for seamless progression into Phase III and commercialization, moving your program forward with clarity and precision.
Validated Shipping Solutions for Global Scale
Deliver with Confidence and Compliance
Phase II trials introduce a new level of logistical complexity. Larger patient populations and multi-site coordination require shipping systems that can handle increased volume while maintaining strict compliance. Cryoport Systems provides a fully-owned fleet of validated shippers designed for cryogenic, ultra-cold, refrigerated, and controlled room temperature transport. Each shipper undergoes our industry-leading Veri-Clean® decontamination and full requalification process, ensuring physical integrity, LN2 capacity, and full system disinfection before every use. Continuous condition monitoring and Cryoportal® logistics management platform integration provide visibility into critical parameters like tilt, shock, temperature, and location, enabling proactive intervention if conditions deviate. Our ISO 21973-certified workflows and risk-based lane qualification strategies reduce variability and protect timelines across global routes.
- Fully-owned, globally deployed fleet for high-volume shipments
- Veri-Clean decontamination and requalification of every shipper to ensure integrity
- Continuous condition monitoring with real-time alerts and Cryoportal integration
- ISO 21973-certified processes for global compliance
- Risk-based lane qualification supporting patient expansion
Phase II sponsors cannot afford delays or deviations when patient-ready materials are in transit. Cryoport Systems delivers shipping solutions that combine validated equipment, advanced monitoring, and global infrastructure to protect your therapy at every step. Our approach ensures transparency, audit-ready documentation, and proactive risk mitigation across every route. By consolidating logistics under one partner, you eliminate fragmentation and gain confidence that every shipment supports your trial objectives. With Cryoport Systems, shipping becomes a competitive advantage that accelerates your path to Phase III and commercialization.
Global Compliance and Risk Mitigation at Scale
Build Confidence for Phase II and Beyond
Phase II trials introduce complexity that magnifies compliance risks. Larger patient populations, multi-site coordination, and international transport require a framework that embeds quality into every step. Cryoport Systems delivers ISO 21973-certified processes and Chain of Compliance® tracking to ensure full traceability across global operations. Our continuous monitoring systems provide near real-time visibility into temperature and location, enabling proactive intervention before deviations impact patient safety. We implement risk-based lane qualification using historical performance data to select routes that minimize variability and delays. By integrating these safeguards early, you create a supply chain that is audit-ready and resilient against disruption. Cryoport Systems transforms compliance from a regulatory requirement into a strategic advantage for global trials.
- ISO 21973-certified workflows for international standards
- Chain of Compliance® for full traceability
- Continuous monitoring with proactive alerts
- Risk-based lane qualification using historical data
- Preventative measures to identify and resolve issues before impact
Compliance during Phase II goes beyond simply meeting regulations and becomes a proactive measure that protects patients and ensures operational continuity as your program scales globally. Cryoport Systems embeds quality and risk mitigation into every process, reducing uncertainty and safeguarding timelines. Our proactive approach identifies potential vulnerabilities before they become costly delays, giving your team confidence in every shipment and every site. By consolidating compliance under one global partner, you eliminate fragmentation and maintain consistency across regions as you advance toward Phase III and commercialization.
Featured Resources

Article
Built for Speed: How Cryoport Systems Delivers Without Compromise
In the life sciences, time isn’t just money, it impacts patient outcomes, regulatory compliance, and even trial integrity. When a temperature-sensitive shipment is delayed due to inventory shipper shortages or logistical bottlenecks, the impact can cascade across an entire program. Unfortunately, these scenarios aren’t theoretical. We hear every day…

Article
The Hidden Risks in Your Supply Chain: How Regulatory Support Can Save Your CGT Program
The temperature-controlled supply chain has become so much more than a straightforward logistics function, especially for advanced therapies. It has quickly evolved into a critical extension of your manufacturing process, your clinical trials, and your path to commercialization. Every step…

Article
Scale Smarter: How Global Supply Chain Centers Amplify CGT Program Growth
Cell and gene therapy (CGT) programs are among the most complex and sensitive operations in the life sciences. From cryopreservation and biostorage to logistics and regulatory compliance, every step in the supply chain can influence product integrity, clinical timelines, and…
Jumpstart your Phase II Clinical supply chain with Cryoport Systems and build a strategy that’s scalable, fully compliant, and ready for every phase ahead.
- IntegriCell® Cryopreservation
Consistent, high-quality leukapheresis starting material.
Learn More >> - BioServices & Biostorage
Integrated solutions to support advanced therapies.
Learn More >> - Consulting & Advisory Services
Our team of experts can help de-risk your logistics.
Learn More >> - Shipping Solutions
Temperature-controlled packaging solutions.
Learn More >> - Transportation
Transportation of commodities at various temperatures.
Learn More >>
Cryoport Systems is Enabling the Outcome™ through our unparalleled supply chain platform.
Offering a comprehensive scope of robust supply chain management solutions and trusted logistics.
Cryoport Systems was established by a team of doctors dedicated to serving the life sciences through expert logistics support. Today, our partnership capabilities have evolved beyond logistics into a robust platform of supply chain solutions that supports every stage and phase of the life sciences research, development, and manufacturing process. Our comprehensive platform of solutions makes Cryoport Systems a standalone vendor that can support all aspects of the temperature-controlled supply chain.
LEARN MORE ABOUT HOW CRYOPORT SYSTEMS IS ENABLING THE OUTCOME™






