Our Solutions

Trusted Solutions for an End-to-End Supply Chain Platform
Integrated supply chain management for a streamlined approach connected by quality and compliance
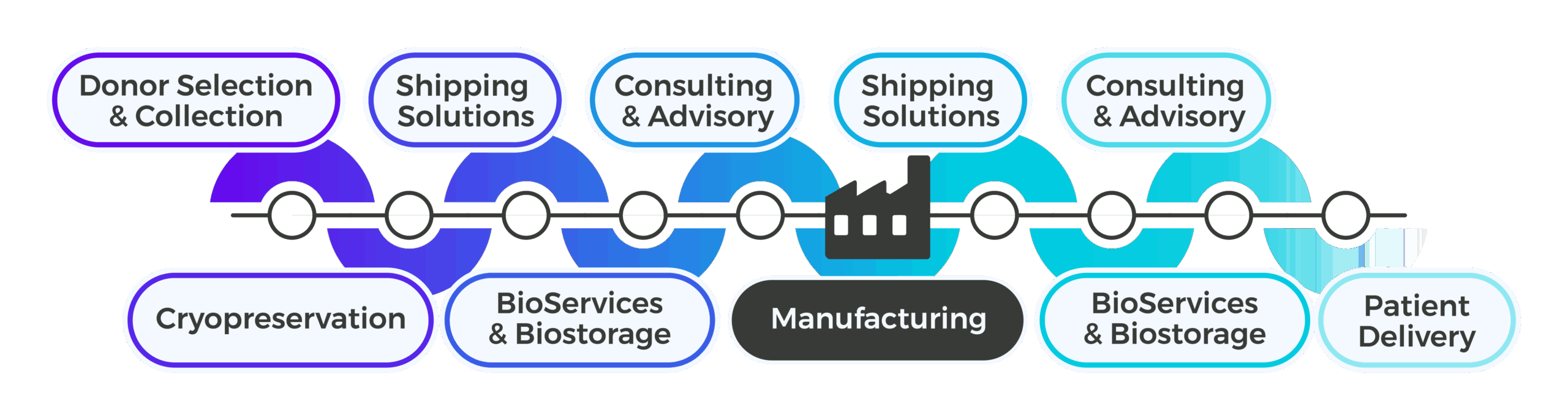
Cryoport Systems delivers a unified supply chain platform that has been purpose-built to support the most demanding applications in the life sciences. From validated, custom-engineered shipping systems that support critical temperature bands to specialized transportation and logistics, from IntegriCell® cryopreservation services to comprehensive BioServices and biostorage, and integrated consulting and advisory services to support regulatory filings and global expansion, every component is engineered to protect the integrity of sensitive biological materials at every stage.
Our platform provides controlled, monitored conditions with industry-leading quality and compliance embedded at every step. Every process is aligned with ISO 21973-certified workflows alongside robust data governance and our proprietary Chain of Compliance® for audit-ready data and documentation. We help reduce variability and risk with flexible and scalable solutions that meet the specific needs of cell therapy, gene therapy, biologics, animal health, and reproductive medicine organizations. Whether you are scaling a clinical program or stabilizing commercial operations, our solutions adapt to your risk profile and regulatory pathway. Cryoport Systems is your single-vendor solution to connect every link in your supply chain with confidence.
Single-Vendor, Integrated Supply Chain Platform
Seamless supply chain management for end-to-end, temperature-controlled services
Cryoport Systems has built a comprehensive supply chain platform that is custom-designed as a connected ecosystem that brings together core capabilities, protecting materials through every handoff both upstream and downstream of manufacturing. Each capability is validated to perform on its own and optimized to perform best as part of a single, integrated workflow. Together, the full scope of supply chain management capabilities provided within a single partnership streamlines vendor management and reduces risk while shortening timelines and providing the flexibility to scale with your program from clinical through commercial.
Custom-Engineered Shipping Systems
Purpose-built solutions for every temperature band, supported by uncompromising quality and process control.
Cryoport Systems offers the industry’s most advanced temperature-controlled shipping solutions, designed specifically for the unique requirements of the life sciences. Whether you’re moving cellular starting material, high-value biologics, or finished therapies, our integrated systems protect your commodities across the full temperature spectrum from cryogenic (-150°C and below), to ultra cold (-60°C to -80°C), refrigerated (2°C to 8°C), and controlled room temperature (15°C to 25°C).
Each system is backed by purpose-built packaging, continuous condition monitoring, and global logistics support, all connected through the Cryoportal® Logistics Management Platform and supported by 24/7/365 oversight. With our combination of proprietary technology, rigorous processes, and deep expertise backed by a track record of success that spans two decades and more than a million successful shipments, Cryoport Systems enables the safe, compliant transport of critical materials at every phase of development.
Logistics and Transportation
Specialized logistics and Cryoshuttle® services designed around your timelines and shipping lanes
When it comes to shipping sensitive materials, Cryoport Systems’ transportation services can be trusted every step of the way. Our logistics capabilities meet the transportation needs of a wide range of materials for the biopharmaceutical, reproductive medicine, and animal health markets. We proudly provide the safe transportation of commodities at temperatures including cryogenic, ultra-cold (dry ice), 2-8˚C, and controlled room temperature. For assistance in moving a single sample across town or shipping an entire fleet of sensitive material from one country to another, you can rely on us and our logistics partners to transport your commodities with their integrity intact.
Additionally, our Cryoshuttle® pickup and delivery service provides local sensitive material transportation within proximal distance to select Cryoport Systems Global Logistics Centers and Global Supply Chain Centers. For materials that need to be shipped internationally, our team of knowledgeable experts can help coordinate customs clearance across borders. Whether it’s domestic or global sensitive material handling, we can accommodate your temperature-controlled supply chain needs.
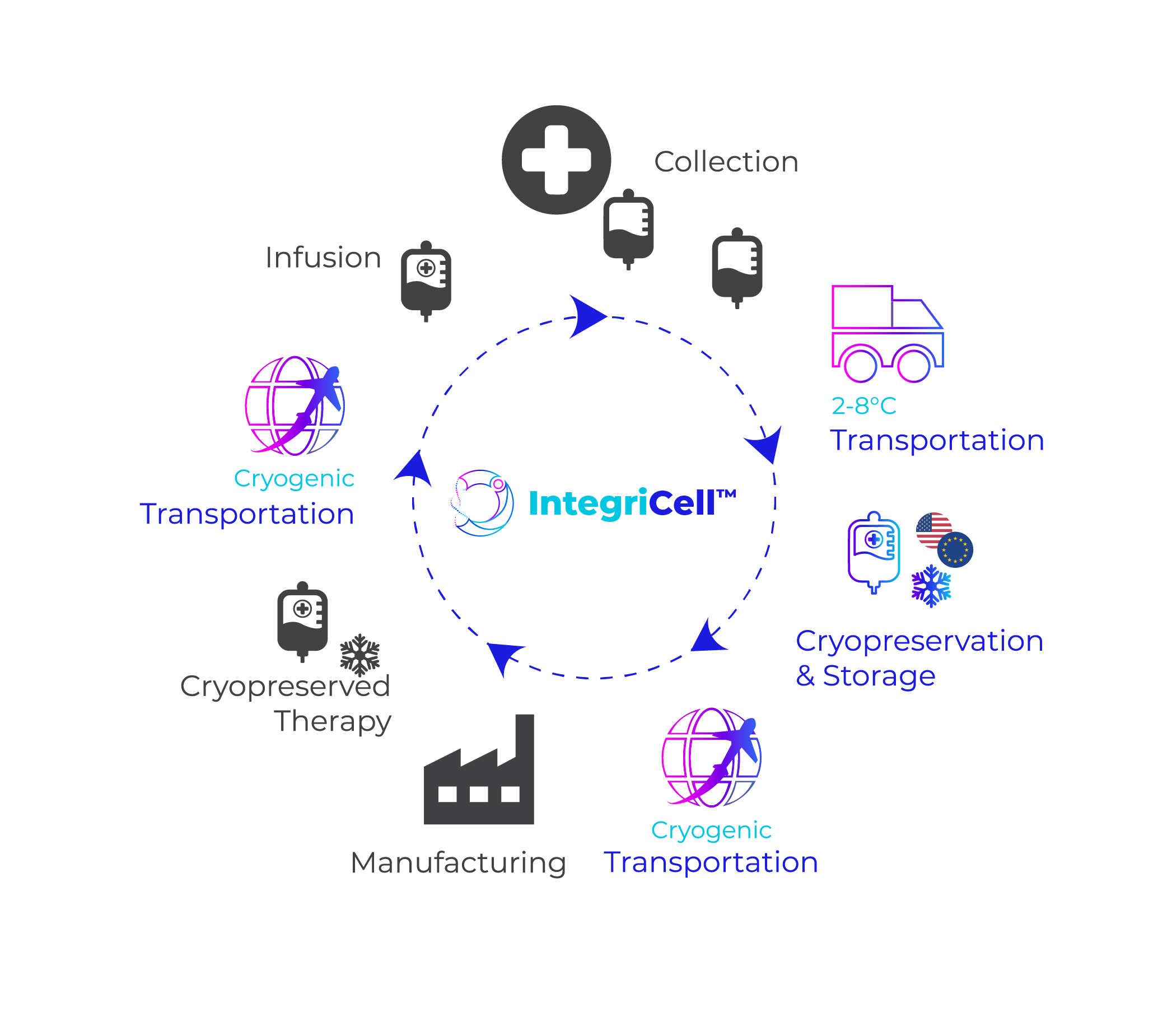
IntegriCell® Cryopreservation
Ensuring unmatched quality, consistency, and reliability for advanced cell therapies
IntegriCell® cryopreservation services from Cryoport Systems optimize leukapheresis-derived cell therapies by enhancing the safety, quality, and viability of manufacture-ready cryopreserved leukopaks. Our state-of-the-art cryopreservation process, integrated with our end-to-end global temperature-controlled supply chain platform, provides unmatched reliability, efficiency, and scope. Our standardized protocols meet the highest compliance standards, ensuring consistent quality and addressing product stability issues associated with fresh donor-derived cellular material for an optimized approach that enhances manufacturing efficiency and streamlines operations.
Comprehensive, Integrated BioServices
A global network of flexible, integrated services that help drive efficiency and scale
Cryoport Systems’ BioServices offering is designed to provide comprehensive and compliant support for the unique needs of biologics and advanced therapy development and commercialization. Our services extend beyond logistics to encompass critical elements such as biostorage, kit production, secondary packaging and labeling, clinical sample management, and regulatory and QP services. This holistic approach ensures that every component of your supply chain is optimized for quality and security with the flexibility you need as your program scales.
By integrating BioServices into our end-to-end platform, we help sponsors, CROs, and CDMOs alike streamline operations, reduce risk, and maintain compliance across global markets. Whether you are in early-phase development or scaling for commercial launch, our solutions are tailored to meet the stringent requirements of cell therapy, gene therapy, and biologics programs with integrated support within our end-to-end supply chain platform approach.
Consulting and Advisory Support
Expert guidance and customized solutions for life sciences logistics excellence
Cryoport Systems’ Consulting Services are designed to provide life sciences organizations with unparalleled expertise and support across their temperature-controlled supply chain. Our consulting practice translates regulatory expectations into practical, workable plans. Our team of industry-leading experts partners with you to create high-quality, compliant solutions that address complex supply chain challenges, streamline clinical trial management, and mitigate risks at every stage.
Our consulting and advisory services support your regulatory requirements, with FDA-required studies for shipping risk assessment, shipping lane qualification, and packaging performance validations. We can support specific program needs at every stage of development from pre-clinical, through clinical trials, and into commercialization with scalable support as you expand into new geographies and your shipping lanes grow more complex. Our team of experts draws on our track record that spans two decades and encompasses more than a million successful shipments to empower your program with robust, reliable, and risk-mitigating solutions.
Ready to get started? Let’s talk!
Support for Every Stage of Development.
Cryoport Systems provides a comprehensive range of integrated temperature-controlled supply chain solutions. Find your perfect solution by clicking through the dropdown below.
Select development phase:

Applications Across the Life Sciences
Purpose-built support for cell therapy, gene therapy, biologics, animal health, and reproductive medicine.
-
Cell Therapy
-
Gene Therapy
-
Biologics
-
Animal Health
-
Reproductive Medicine
At Cryoport Systems, quality is engineered into every process. From ISO 21973 certification to Chain of Compliance® traceability and Veri-Clean® validated decontamination, we set the standard.
- IntegriCell® Cryopreservation
Consistent, high-quality leukapheresis starting material.
Learn More >> - BioServices & Biostorage
Integrated solutions to support advanced therapies.
Learn More >> - Consulting & Advisory Services
Our team of experts can help de-risk your logistics.
Learn More >> - Shipping Solutions
Temperature-controlled packaging solutions.
Learn More >> - Transportation
Transportation of commodities at various temperatures.
Learn More >>
Cryoport Systems is Enabling the Outcome™ through our unparalleled supply chain platform.
Offering a comprehensive scope of robust supply chain management solutions and trusted logistics.
Cryoport Systems was established by a team of doctors dedicated to serving the life sciences through expert logistics support. Today, our partnership capabilities have evolved beyond logistics into a robust platform of supply chain solutions that supports every stage and phase of the life sciences research, development, and manufacturing process. Our comprehensive platform of solutions makes Cryoport Systems a standalone vendor that can support all aspects of the temperature-controlled supply chain.
LEARN MORE ABOUT HOW CRYOPORT SYSTEMS IS ENABLING THE OUTCOME™