Supply Chain Archives
10/15/2025
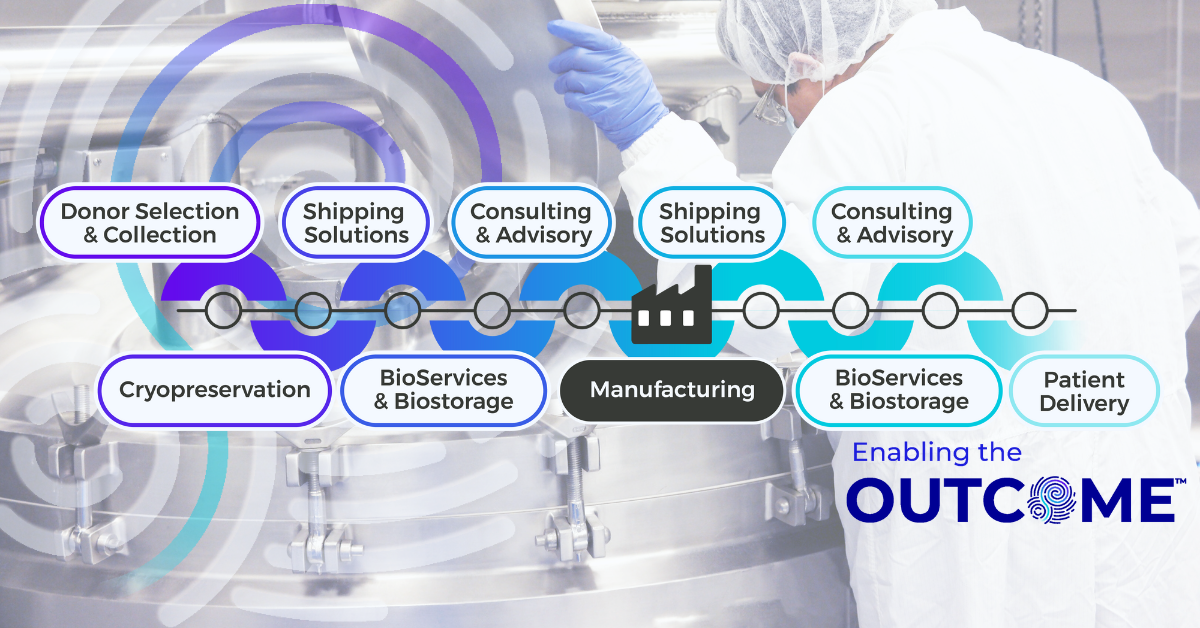
The Connected Chain: Why Supply Chain Integration Matters in Advanced Therapy Manufacturing
Contract Development and Manufacturing Organizations (CDMOs) are at the heart of the advanced therapy ecosystem, managing complex, high-stakes programs that demand speed and compliance. As therapies move from early development into clinical trials and through to commercialization, every stage of manufacturing, packaging, biostorage, and final drug delivery need to align perfectly for seamless execution. Yet in many cases, these functions are managed by multiple vendors, each operating in isolation. The result is a fragmented supply chain that increases risk while slowing execution due to layers of operational burden.Categories
- All (62)
- Industry Insights (20)
- Managing the Cold Chain (50)
- Navigating Logistics (27)
- Patient Access and Awareness (8)
Tags
- Asia
- ATMPs
- Biologics
- BioServices
- Biostorage
- CDMO
- CDMOs
- Cell Therapy
- Clinical Trials
- Cold Chain Management
- Commercialization
- CROs
- Cryopreservation
- Europe
- Fragmentation
- Gene Therapy
- Global Logistics Tracking
- Global Supply Chain Management
- International Shipping
- North America
- Risk Mitigation
- Standards and Compliance
- Supply Chain Management