Support for Phase I Clinical Programs

Clinical Phase I Solutions for Advanced Therapy Developers
Secure your first-in-human trials with scalable, compliant supply chain support
Phase I trials mark a critical milestone as your therapy moves from concept to patient care, a milestone that means the margin for error disappears. Every shipment, every process, every decision… everything you do needs to protect patient safety and uphold regulatory standards. Cryoport Systems partners with you from the earliest stages to deliver a supply chain that is IND-ready and globally scalable, designed now for continuity into later phases. Our integrated, end-to-end supply chain platform eliminates fragmentation and reduces operational burden, allowing you to focus on science while we manage complexity.
With fully-owned shipping fleets, integrated condition monitoring, and ISO 21973-certified processes, we ensure your materials are protected across every mile. From cryopreservation of leukapheresis-derived starting material to custom kit building for sample collection and manufacturing, our solutions standardize workflows and mitigate risk. By working with Cryoport Systems, you gain a partner who understands the stakes and delivers confidence for your first patients and every milestone ahead.
Your Clinical Supply Chain, Built for Phase I Success
Comprehensive solutions that protect patients and accelerate progress
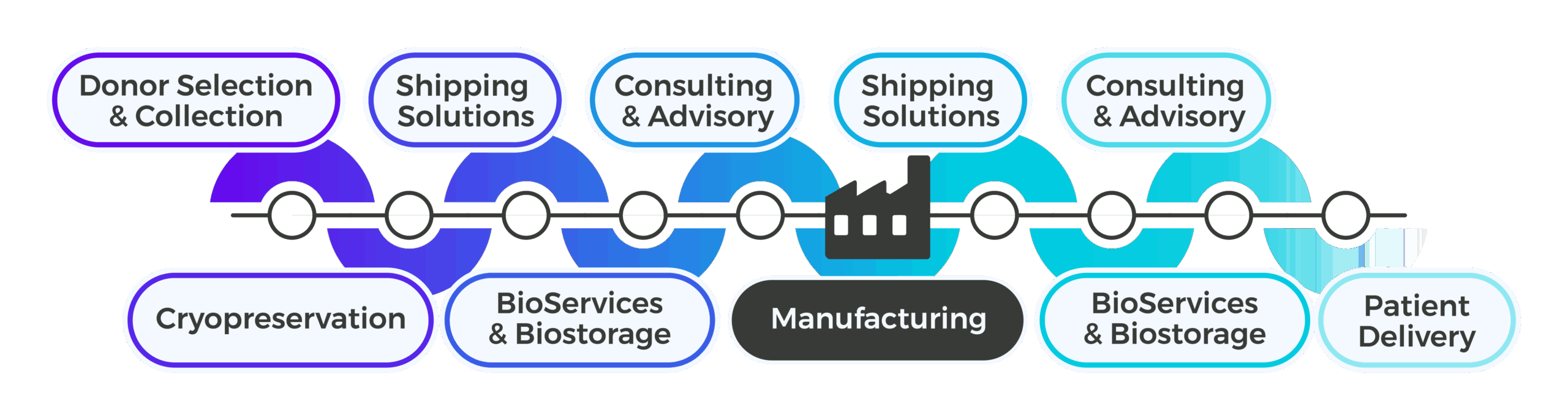
Support Upstream of Manufacturing
Build Standardized Processes Before First Patient Enrollment
Phase I trials demand upstream strategies that anticipate regulatory scrutiny and future scalability. Cryoport Systems partners with you to design GMP-aligned processes and workflows that eliminate variability and ensure reproducibility across sites. Our experts help you standardize processes for leukapheresis collection and manufacturing kits, reducing risk and avoiding costly rework as you progress. By integrating cryopreservation and biostorage planning early, you create a seamless bridge between manufacturing and patient delivery. Global facility access and uniform procedures across regions allow you to support multi-site trials without compromising compliance.
- GMP and ISO 21973 compliance before your first patient
- Standardized kit design for collection and manufacturing
- End-to-end integration that includes cryopreservation, logistics, and biostorage
- Global facility access to support multi-site trials
- Advisory support for proactive risk mitigation and regulatory filings
Early-phase trials leave no room for inefficiency or compliance gaps. By partnering with Cryoport Systems, you gain a supply chain foundation that anticipates IND requirements and scales seamlessly as your program grows. Our upstream strategies eliminate variability across sites, reduce operational burden, and prevent costly rework later in development. This proactive approach means your team can focus on patient enrollment and scientific milestones while we ensure every process is standardized and audit-ready. With Cryoport Systems, upstream planning becomes a strategic advantage that accelerates your path to Phase II and beyond.
Support Downstream of Manufacturing
Secure Transport for First-in-Human Trials
Once manufacturing is complete, downstream logistics become the lifeline of your clinical program. Cryoport Systems provides validated shipping systems, Smartpak™ condition monitoring, and Chain of Compliance® tracking to ensure every shipment meets regulatory standards. Our fully-owned shipping system fleet eliminates capacity constraints, while Veri-Clean® decontamination and industry-leading requalification processes guarantee shipper integrity for every use. ISO 21973-certified processes and risk-based lane selection reduce variability and protect timelines. Cryoshuttle® adds rapid local delivery for first and last mile, adding another layer of protection for sensitive materials.
- Integrated Smartpak™ for continuous monitoring of every shipment throughout transit
- Chain of Compliance® enables audit-ready data for IND readiness
- Veri-Clean® decontamination and requalification of every shipping system before every use
- ISO 21973-certified workflows and processes for industry-leading compliance and quality
- Cryoshuttle® for specialized local pickup and delivery available in select locations
Downstream logistics during Phase I are mission-critical. Every shipment represents irreplaceable patient material, and delays or deviations can jeopardize trial integrity. Cryoport Systems delivers a proven framework that brings together validated equipment, continuous monitoring, and proactive risk mitigation alongside critical services like BioServices and biostorage to keep your program on track. Our integrated approach reduces handoffs and ensures full visibility with audit-ready documentation, reducing stress and uncertainty for your team. Partnering with Cryoport Systems provides peace of mind that your patient-ready materials are being cared for by a full team of experts, backed by a track record of success that spans decades.
Cryopreservation that Safeguards Starting Material
Standardize Leukapheresis for Consistency and Scale
Cryopreservation during Phase I is critical to preserving cell integrity and therapeutic efficacy. Cryoport Systems’ IntegriCell® solution provides validated protocols and expert guidance to ensure consistency across sites and batches. By implementing our Automated Closed Process early, you reduce variability and enable smooth tech transfer into GMP environments. Our team supports process validation and equipment selection, ensuring compliance and reproducibility.
- Validated cryopreservation protocols for leukapheresis-derived starting materials
- Proprietary, validated Automated Closed Process for industry-leading consistency
- Seamless GMP integration
- Enhanced sample integrity and viability
Starting material is the foundation of your therapy, and its integrity cannot be compromised. Cryoport Systems helps you implement cryopreservation strategies that protect viability and standardize processes across all trial sites. This consistency reduces risk, supports reproducibility, and extends manufacturability as you scale. By building these safeguards into Phase I, you avoid costly disruptions and ensure patient safety remains uncompromised.
Integrated BioServices for Clinical Trials
Standardize Collection, Storage, and Management
Phase I trials require precise coordination of sample collection, storage, and management. Cryoport Systems’ BioServices provide GMP-ready biostorage combined with standardized sample collection kits and manufacturing kits to reduce variability across sites. Our clinical sample management capabilities ensure full traceability and integrity throughout the trial lifecycle. With global facilities and continuous monitoring, you gain confidence that every sample meets compliance standards.
- GMP-ready biostorage facilities worldwide
- Standardized sample collection kits and manufacturing kits reduce variability
- Clinical sample management for full lifecycle oversight
- Continuous monitoring and audit-ready documentation
- Integrated logistics and cryopreservation support
Managing samples across multiple sites during Phase I can quickly become complex and susceptible to error. Cryoport Systems simplifies this challenge by delivering integrated BioServices that standardize collection, storage, and handling from the start. Our solutions reduce variability and maintain chain of custody while providing audit-ready documentation for regulatory submissions. This level of control both ensures patient safety and accelerates trial timelines. With Cryoport Systems, BioServices become a strategic asset that supports your program through every milestone.
Expert Guidance for IND and Phase I Success
Navigate Regulatory and Operational Complexity
Phase I introduces new layers of complexity, from regulatory requirements to multi-site coordination. Cryoport Systems’ consulting and advisory services provide strategic insights to help you plan for scalability, compliance, and risk mitigation. Our experts collaborate with your team to proactively mitigate common risks, select optimal shipping lanes, and anticipate regulatory expectations. We also deliver FDA-required shipping risk assessments, lane qualifications, and packaging validations to pre-clear your commercial runway.
- Risk-based logistics planning
- FDA-required risk assessments and lane qualifications
- Packaging validation for IND readiness
- Insights from global program experience
Phase I is where regulatory expectations begin to shape your operational reality. Cryoport Systems helps you navigate this complexity with proactive planning and expert guidance. Our consulting and advisory services reduce uncertainty and prevent compliance gaps, positioning your program for smooth progression into later phases. By leveraging our experience gained across thousands of advanced therapy programs and more than a million successful deliveries, you gain a partner who understands the nuances of early-phase logistics and delivers solutions tailored to your needs.
Validated Shipping Solutions for Phase I Trials
Deliver with Confidence and Compliance
Shipping patient-ready materials requires precision and reliability. Cryoport Systems provides a fully-owned fleet of validated shippers designed for cryogenic, ultra-cold, refrigerated, and controlled room temperature transport. Each shipper undergoes Veri-Clean® decontamination and full shipping system requalification before every use, ensuring compliance and safety for every shipment. Our continuous monitoring and Chain of Compliance® tracking give you full visibility and audit-ready documentation. With ISO 21973-certified processes and risk-based lane selection, you can trust that your materials will arrive intact and on time.
- Industry’s largest, fully owned fleet of shipping systems
- Custom-engineered shipping solutions for the life sciences
- Veri-Clean decontamination and requalification processes
- Continuous condition monitoring and audit-ready documentation
- ISO 21973-certified processes and industry-leading quality standards
- Risk-based lane selection supporting patient expansion
Every shipment in Phase I carries irreplaceable patient material, and failure is not an option. Cryoport Systems delivers shipping solutions that combine validated equipment, rigorous decontamination and requalification processes, and integrated continuous monitoring to protect your therapy at every step. Our integrated processes and Cryoportal® logistics management system provide transparency and compliance assurance, reducing risk and enabling faster decision-making. By choosing Cryoport Systems, you secure a logistics framework that supports patient safety and accelerates your path to success.
Compliance Built into Every Step
Protect Patient Safety and Program Integrity
Regulatory compliance and risk mitigation are central to Phase I success. Cryoport Systems embeds quality into every process, from cryopreservation to biostorage and shipping. Our ISO 21973 certification, Chain of Compliance® tracking, and continuous monitoring provide a robust framework for audit readiness. By implementing risk-based strategies early, you avoid costly delays and ensure smooth progression through trial phases.
- ISO 21973-certified processes
- Chain of Compliance® audit trail
- Continuous monitoring for patient safety
- Proactive issue identification and resolution
Compliance and risk management during Phase I safeguard both patient safety and program credibility. Cryoport Systems integrates quality and risk mitigation into every step of your supply chain, ensuring audit readiness and operational continuity. Our proactive approach identifies potential issues before they impact timelines, reducing stress and uncertainty for your team. With Cryoport Systems, compliance becomes a competitive advantage that accelerates your journey toward approval.
Featured Resources

Poster Presentation
Cryo-processed Leukapheresis Using Automated Closed System
This poster presentation video demonstrates how automation, standardization, and data-driven workflows can help you reduce variability and build confidence in your cell therapy programs. From standardized cryopreservation to integrated supply chain solutions, Cryoport Systems is Enabling the Outcome™ to future-proof your advanced therapy programs.

Article
Protecting the Foundation of Biologics with Flexible, Scalable Cell Banking
For any biotherapeutic program, your cell bank is the foundation of your manufacturing process. Master cell banks (MCBs), working cell banks (WCBs), and research cell banks (RCBs), represent years of development work and intellectual property. They are irreplaceable assets, and the security of these assets is directly tied to…

Article
Scale Smarter: How Global Supply Chain Centers Amplify CGT Program Growth
Cell and gene therapy (CGT) programs are among the most complex and sensitive operations in the life sciences. From cryopreservation and biostorage to logistics and regulatory compliance, every step in the supply chain can influence product integrity, clinical timelines, and the scalability of…
Jumpstart your Phase I Clinical supply chain with Cryoport Systems and build a strategy that’s scalable, fully compliant, and ready for every phase ahead.
- IntegriCell® Cryopreservation
Consistent, high-quality leukapheresis starting material.
Learn More >> - BioServices & Biostorage
Integrated solutions to support advanced therapies.
Learn More >> - Consulting & Advisory Services
Our team of experts can help de-risk your logistics.
Learn More >> - Shipping Solutions
Temperature-controlled packaging solutions.
Learn More >> - Transportation
Transportation of commodities at various temperatures.
Learn More >>
Cryoport Systems is Enabling the Outcome™ through our unparalleled supply chain platform.
Offering a comprehensive scope of robust supply chain management solutions and trusted logistics.
Cryoport Systems was established by a team of doctors dedicated to serving the life sciences through expert logistics support. Today, our partnership capabilities have evolved beyond logistics into a robust platform of supply chain solutions that supports every stage and phase of the life sciences research, development, and manufacturing process. Our comprehensive platform of solutions makes Cryoport Systems a standalone vendor that can support all aspects of the temperature-controlled supply chain.
LEARN MORE ABOUT HOW CRYOPORT SYSTEMS IS ENABLING THE OUTCOME™






