Biologics

Expansive solutions for biologics distribution
Our comprehensive supply chain platform encompasses a full range of risk-mitigating solutions
Distributing biologics requires meticulous planning and preparation. You must consider procedures like the cryogenic storage and transport of master cell banks, the bulk transfer of API (Active Pharmaceutical Ingredients), and the distribution of the products to the end user. Each of these steps require specific storage, packaging, and transportation considerations that are rarely accomplished in-house. We can help supplement your needs with our tailored services.
De-fragmenting the temperature-controlled supply chain for biologics
At Cryoport Systems, our integrated platform of solutions can support you through the entire supply chain, from temperature-controlled biostorage and the transportation of master cell banks to -80°C transfer of APIs.
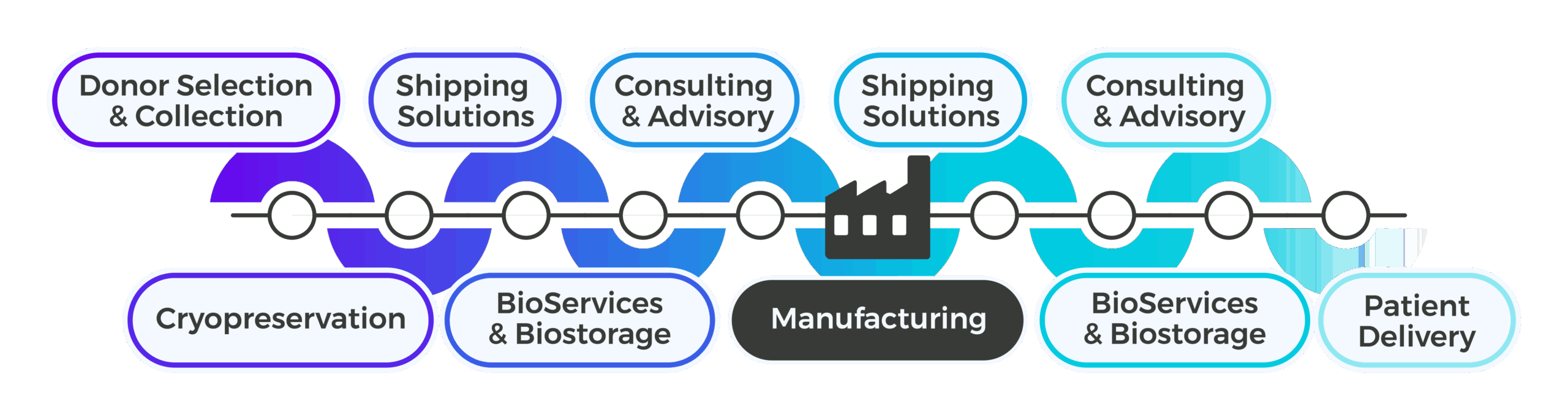
Support Upstream of Manufacturing
Upstream of manufacturing, Cryoport Systems helps biologics developers establish consistent, compliant logistics for critical raw materials, biospecimens, and intermediates. Whether supporting early-phase biologic discovery or clinical-grade material collection, we deliver standardized collection kits and validated shipping systems directly to sites, ensuring Chain of Compliance® and minimizing variability. Materials are transported under strict environmental controls to designated processing or storage facilities, including our IntegriCell™ cryopreservation platform when temperature-sensitive preservation of leukapheresis-derived cellular material is required. With integrated consulting and advisory services for packaging qualification, lane validation, and global regulatory alignment, we streamline upstream workflows to reduce supply chain complexity, accelerate readiness, and maintain the integrity of sensitive biologic inputs.
Support Downstream of Manufacturing
Downstream of manufacturing, Cryoport Systems provides end-to-end logistics, BioServices and biostorage, and consulting and advisory support that enables the reliable, scalable delivery of biologic products to clinical and commercial destinations. Our validated shipping systems maintain critical temperature ranges for proteins, antibodies, vaccines, and other biologics during global transit. Through our BioServices platform, we offer pick, pack, label, and distribution workflows that can be tailored to product and trial needs, while custom secondary packaging solutions ensure safe handling of specialized containers and components. Our global team supports ongoing trial expansion, lane qualifications, and QP release in the EU, enabling seamless geo-expansion. By consolidating logistics and supply chain management services under one partner, biologics developers eliminate coordination challenges, protect product quality, and gain the flexibility to scale efficiently.
Support for CDMOs
Cryoport Systems supports CDMOs working across the biologics development lifecycle, from preclinical material preparation to late-stage commercial manufacturing, by delivering a unified, compliance-driven supply chain management platform. Biologics programs face distinct challenges: sensitive payloads, strict cold chain requirements, and increasing demand for batch consistency across diverse clinical and commercial settings. We address these challenges head-on with validated, custom-engineered shipping systems, end-to-end traceability, and integrated BioServices and biostorage options that reduce risk and support manufacturing excellence. Whether managing proteins, monoclonal antibodies, or vaccine components, CDMOs gain greater control, standardization, and scalability through our single-vendor model.
Cryoport Systems is the trusted partner for biologics CDMOs seeking to reduce variability, ensure regulatory readiness, and scale efficiently.
We offer tailored support for every stage of the manufacturing process, including raw material handling, intermediate storage, and just-in-time delivery to fill/finish or packaging lines. Our Chain of Compliance® platform captures full shipment history and condition data, enabling batch-level documentation and audit-ready oversight. CDMOs also benefit from our global network of strategically located facilities, Qualified Person (QP) services for EU imports, and consulting expertise that helps proactively qualify shipping lanes and align operations with evolving regulatory expectations. With Cryoport Systems, biologics CDMOs can meet client demands while maintaining the integrity and reliability required in a tightly regulated space.
Support for CROs
Clinical trials for biologics demand exceptional coordination and precision, especially when working across multiple geographies and cold chain requirements. Cryoport Systems partners with CROs to manage the logistical complexity of biologics studies, helping to ensure that investigational products, clinical samples, and trial kits are delivered on time and in compliance. By centralizing logistics, packaging, biostorage, and consulting under one partner relationship, we reduce the risk of material loss, delayed dosing, and other hurdles. Our end-to-end supply chain platform brings much-needed standardization and predictability to CRO operations supporting protein, antibody, and vaccine-based studies.
Cryoport Systems helps CROs streamline biologics trial execution with greater speed, reliability, and regulatory alignment.
We offer temperature-validated, custom-engineered shipping systems that align with biologic stability profiles, as well as biostorage to support redundant storage of critical materials like cell banks and just-in-time delivery of materials. Our BioServices teams handle pick, pack, label, and distribution tailored to study protocols, and our consulting and advisory services provide proactive support for shipping lane validation, customs requirements, and risk mitigation. With our global footprint and advanced Chain of Compliance® visibility, CROs can manage complex trial logistics while focusing on their core mission of driving clinical success and accelerating sponsor timelines.
Support for Therapeutic Developers
Biologics developers operate in a fast-paced, highly regulated environment where even minor disruptions can quickly turn into costly delays. Cryoport Systems provides a robust and flexible end-to-end supply chain foundation tailored to the needs of biologics sponsors, whether advancing a monoclonal antibody, a therapeutic protein, or a next-generation vaccine. From early-stage clinical material movement to commercial launch logistics, our integrated platform enables developers to reduce complexity, accelerate development timelines, and protect the integrity of temperature-sensitive materials at every touchpoint.
Cryoport Systems empowers biologics developers to move from R&D to commercialization with a unified, audit-ready supply chain built for global scale.
We provide validated cold chain solutions, global biostorage, and customized packaging configurations that ensure temperature stability and protect product integrity from batch release to delivery. Our consulting and advisory teams help sponsors navigate the nuances of global distribution and prepare for regulatory audits. Developers also benefit from real-time visibility, compliance documentation, and the ability to scale quickly across clinical phases and commercial territories. With Cryoport Systems as your supply chain partner, biologics developers can focus on innovation, knowing the logistics are built for success.
Support for Every Stage of Development.
Cryoport Systems provides a comprehensive range of integrated temperature-controlled supply chain solutions. Find your perfect solution by clicking through the dropdown below.
Select development phase:

Leverage a platform with a proven track record of compliance, reliability, and innovation.
- IntegriCell™ Cryopreservation
Consistent, high-quality leukapheresis starting material.
Learn More >> - BioServices & Biostorage
Integrated solutions to support advanced therapies.
Learn More >> - Consulting & Advisory Services
Our team of experts can help de-risk your logistics.
Learn More >> - Shipping Solutions
Temperature-controlled packaging solutions.
Learn More >> - Transportation
Transportation of commodities at various temperatures.
Learn More >>
Cryoport Systems is Enabling the Outcome™ through our unparalleled supply chain platform.
Offering a comprehensive scope of robust supply chain management solutions and trusted logistics.
Cryoport Systems was established by a team of doctors dedicated to serving the life sciences through expert logistics support. Today, our partnership capabilities have evolved beyond logistics into a robust platform of supply chain solutions that supports every stage and phase of the life sciences research, development, and manufacturing process. Our comprehensive platform of solutions makes Cryoport Systems a standalone vendor that can support all aspects of the temperature-controlled supply chain.
Learn More about how Cryoport Systems is Enabling the Outcome™