Establish Stability from the Start with Frozen Starting Materials for Cell Therapy Development
For many years, cell therapy programs defaulted to fresh leukapheresis-derived starting material. The assumption was that if you could minimize the time from collection to manufacturing, you would maximize viability. As a result, fresh apheresis became not only a scientific preference but an operational tradition, shaping how clinical teams would schedule donors, how manufacturing suites would allocate capacity, and how programs would structure day-to-day execution. Program leaders treated any deviation from “fresh” as a risk to be justified rather than a choice to be evaluated. But reality keeps getting in the way.
As the field has matured and the pressures around reproducibility, scale, and meeting changing regulatory expectations have increased, the limitations of this legacy model are growing more pronounced. Fresh material, while appealing conceptually, introduces significant operational and scientific variability just as programs seek greater control. Increasingly, “fresh” is no longer the default definition of “better.” Teams are feeling the impact of how fragile fresh material really is. How a single delay can unravel an entire manufacturing plan, how donor-to-donor variability slips when tight processing windows are skewed, how difficult it becomes to truly compare batches when the starting point keeps shifting. The time sensitivity and coordination required for working with fresh material introduce unpredictability that directly affects viability, variability, and other critical parameters that only grow more problematic as programs progress.
Frozen starting material has become the counterpoint to those headaches. It’s consistent. It’s predictable. It decouples collection from manufacturing, adding flexibility and clearing roadblocks. And when cryopreservation of fresh leukapheresis is implemented through a GMP-aligned automated closed process (ACP) that integrates into the full end-to-end supply chain platform, it lowers risk and increases predictability, creating a more scalable path for early-phase programs.
The Structural Limitations of Fresh Leukapheresis
The challenges associated with fresh starting material create scheduling complexity and logistical inconveniences, sure. But beyond these structural roadblocks, the limitations of working with fresh leukapheresis material are inherent to biological systems and the constraints imposed by real-world operations.
Fresh material begins to change the moment collection is completed. Viability, phenotype, and functional attributes of the collected cells are sensitive to any number of variables, even under tight process controls. Handling inconsistencies, differences in operator technique or collection kits, transport duration, pre-processing wait time… the sheer number of individual factors makes consistency difficult to achieve, especially under the tight timelines required for manufacturing cell therapies from living cells. Fresh workflows, out of necessity, compress the entire chain (collection, packaging, transport, intake, and first manufacturing step) into a narrow window that needs to be completed within an ideal 24- to 48-hour period. This forces teams into rigid timelines where any disruption, from weather delays to site staffing constraints, can compromise material integrity or require rescheduling. Because of this, teams routinely prepare for a “fresh day” with an overcommitment of personnel and resources, not because the biology demands it, but because the fragility of the workflow requires it.
These pressures impact operations as well as analytical interpretation and regulatory documentation. When the starting material introduces more variability than the process itself, it becomes difficult to isolate and evaluate true process performance. Drift in cellular viability can be misinterpreted as process variability, for example. Regulatory reviewers seeking comparability across runs or phases can encounter narratives that attribute differences to circumstances and not controlled variables. Fresh material can introduce scientific noise at these critical points where clarity is essential.
Stabilizing Input Variability Through Cryopreservation
Cryopreservation of leukapheresis-derived starting materials, when executed in a controlled and validated, GMP-aligned manner, offers a fundamentally different path. Instead of racing against time, ACP-enabled cryopreservation arrests the state of cells at a defined and characterized point, ideally within 24- to 48-hours of collection. This establishes a stable baseline from which manufacturing can proceed repeatedly and predictably.
With automated processing, controlled-rate freezing, and GMP-aligned workflows, cryopreservation maintains cellular viability and functionality in a standardized and reproducible manner. The output is not a compromise but a stabilized, consistent material that can move into manufacturing and downstream processing without the logistical variability of fresh material. Early-phase teams gain the confidence that comes from consistently prepared inputs, leading to cleaner analytical interpretation and more actionable insights.
Operationally, cryopreserved starting material releases programs from the constraints of donor-aligned scheduling. Manufacturing runs can be planned when the suite is ready and personnel are available. The decoupling of collection and manufacturing increases efficiency and reduces downtime, helping to mitigate the cascading delays that often come with fresh workflows. Programs gain flexibility without sacrificing scientific validity. In fact, they gain additional precision by reducing the batch-to-batch variability that is inherent to fresh material.
As a result, cryopreservation of starting material becomes both a practical and strategic choice, supporting a controlled, iterative process development pathway from pre-clinical work through Phase I and into Phase II with fewer disruptive transitions and scalability built into SOPs from the start.
Integration Treats Cryopreservation as a System, not a Step
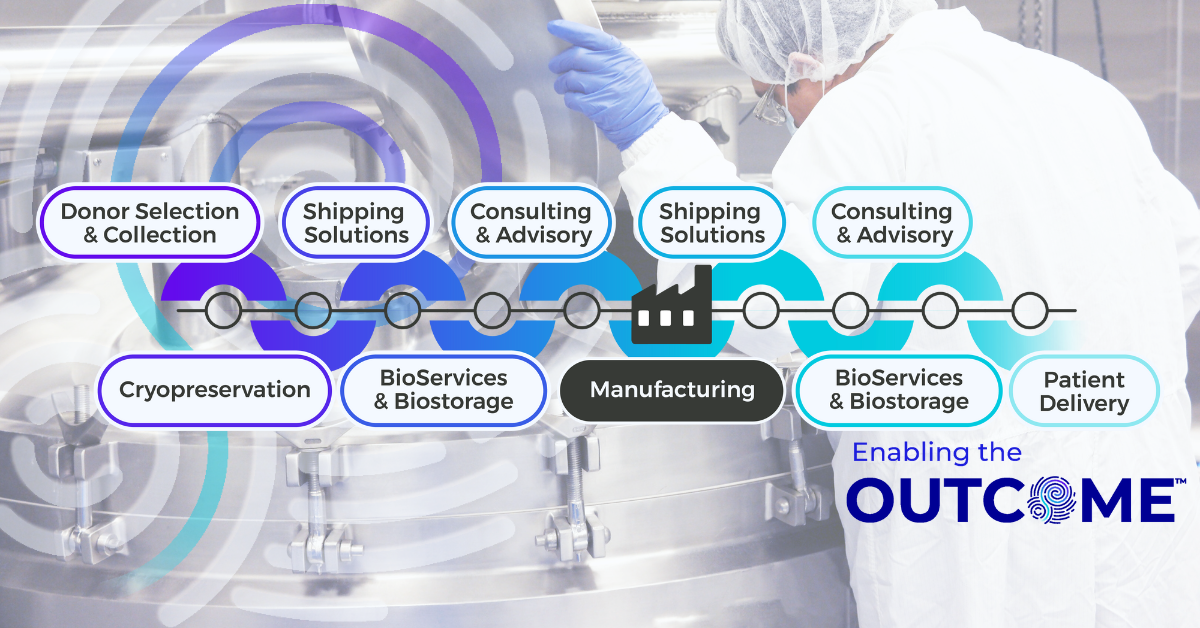
While cryopreservation itself is critical, its reliability as an approach depends on the infrastructure surrounding it. Freezing can be implemented at any point, from collection sites to third-party processors to manufacturing sites. Freezing in a manner that supports regulatory compliance, global distribution, long-term scalability plans, and standardization for controllable consistency? That is fundamentally different. This is where the integrated Cryoport Systems platform approach to supply chain management, bringing together IntegriCell® cryopreservation, BioServices and biostorage, custom-engineered shipping systems, logistics, and continuous monitoring within a single-vendor model, becomes essential.
Cryopreservation cannot be isolated from its upstream and downstream environments. Shipping systems and secondary packaging influence the thermal profile. Shipping lanes influence exposure risk. Storage environments determine stability. Data continuity across handoffs determines how deviations are interpreted. Fragmenting these steps across multiple vendors introduces variability and a higher likelihood of untracked excursions.
Cryoport Systems’ integrated infrastructure eliminates many of these vulnerabilities through unified processes that span our entire global footprint. Collection, manufacturing, and administration kits are standardized within our full suite of BioServices. ACP cryopreservation is executed under GMP-aligned processes that integrate seamlessly with biostorage protocols, shipping systems engineered for cryogenic profiles, and global distribution lanes supported by continuous monitoring and audit-ready Chain of Compliance® documentation. Chain of identity, chain of custody, and chain of condition are maintained within a single framework. Customizable consulting and advisory services produce shipping risk assessments and shipping lane qualifications to support your regulatory filings. All segments of the workflow are documented and controlled together.
For sponsors, this reduces both operational risk and regulatory burden. Programs do not need to reconcile multiple documentation systems or explain discrepancies across a patchwork of vendor solutions. Instead, they present a cohesive and forward-compatible control strategy that shows stability from the moment materials enter the cryopreservation process through final manufacturing and patient administration.
A Stronger Position for Regulatory Review and Investor Evaluation
Increasingly, regulatory agencies and investors evaluate early-phase programs not only on scientific merit but also on operational maturity and risk mitigation as a measure of readiness for scale. Cryopreservation of starting materials (coupled with GMP-compliant biostorage and logistics) strengthens a program’s position all the way around.
From a regulatory standpoint, consistency of input material simplifies comparability assessments, supports clearer batch analyses, and reduces unexplained or unexpected variance in potency and characterization assays. When the starting material is stable and well-documented, reviewers can focus on process performance and not on attempting to detangle process effects from input variability.
From an investor standpoint, an integrated cryopreservation strategy signals long-term thinking. It shows that the program has aligned its early-phase practices with the future demands of multi-site, multi-geography expansion. It demonstrates that operational risks, often the cause of delayed milestones or increased burn, have been proactively mitigated. In a landscape where capital efficiency is being more scrutinized than ever, the ability to show a predictable operational path carries significant weight.
The use of cryopreserved, well-controlled starting material aligns the development narrative with the expectations of both groups. As a result, the program is seen as not only scientifically compelling but also operationally robust, prepared for what comes next as it progresses into later phases.
A Predictable Development Path Builds the Foundation for Future Scale
Programs that adopt a cryopreservation workflow from the outset experience a shift in how development progresses. The day-to-day operations become more reliable, and batch-to-batch data becomes more interpretable. Manufacturing departments can proactively plan suite utilization rather than constantly adjusting last-minute around collection scheduling. Clinical teams can manage collections separate from manufacturing, and workflows become predictable and streamlined.
Most importantly, the program moves through each phase with fewer forced transitions. The same cryopreservation strategy that stabilizes early-phase material supports Phase II and Phase III scale-up without major systemic changes. Documentation and consistency remain stable as volume increases and geographies expand. This continuity reduces risk while accelerating timelines, ultimately creating a smoother path toward commercialization.
Fresh starting material, although long-considered the standard, introduces operational fragility and scientific variability that can hinder early-phase programs and complicate long-term development. Frozen starting material, when executed under an ACP and within an integrated end-to-end supply chain, offers a more stable and scalable alternative.
IntegriCell cryopreservation from Cryoport Systems enables sponsors to adopt this model from day one, positioning programs for greater predictability and stronger regulatory alignment throughout the clinical phases and into commercialization. Rather than relying on reactive coordination, sponsors build their development strategy on a platform designed for consistency and growth.
The field is moving away from fresh-by-tradition to frozen-by-design. With IntegriCell cryopreservation services, Cryoport Systems provides the infrastructure and operational integration required to make that transition not only feasible, but strategically advantageous.