Supply Chain Archives

01/27/2026
Establish Stability from the Start with Frozen Starting Materials for Cell Therapy Development
For many years, cell therapy programs defaulted to fresh leukapheresis-derived starting material. The assumption was that if you could minimize the time from collection to manufacturing, you would maximize viability. As a result, fresh apheresis became not only a scientific preference but an operational tradition, shaping how clinical teams would schedule donors, how manufacturing suites would allocate capacity, and how programs would structure day-to-day execution. Program leaders treated any deviation from “fresh” as a risk to be justified rather than a choice to be evaluated. But reality keeps getting in the way.
01/27/2026
How Defragmenting the Pre-Clinical Supply Chain De-Risks Downstream Operations
Every stage of the supply chain including cryopreservation, logistics, and BioServices and biostorage, must align to ensure a seamless transition from pre-clinical work into Phase I trials. However, early-stage teams often select multiple vendors who each handle one piece of the process because it feels faster or more cost-effective in the moment. This approach, however, builds fragmented supply chains that introduce gaps as programs scale. And when those gaps surface, teams must devote valuable time and effort simply to get back on track.
12/19/2025
Expert Q&A: Is Standardization the Bottleneck or Breakthrough to Building Scalable and Cost-Effective CGT Programs?
Cell and gene therapy (CGT) companies are under constant pressure to do more while hitting milestones earlier. Demonstrate manufacturability. Ensure regulatory readiness. Design for scalability and patient access. And do all of this with more constrained resources and increasingly aggressive timelines. Building on our recent panel discussion, we gathered extended perspectives from Dr. Don Fink (Dark Horse Consulting Group), Audrey Greenberg (Mayo Venture Partners), Dr. Dominic Clarke (Cryoport Systems), and Kurtis Carlisle (Cabaletta Bio).
12/17/2025
2025 Year in Review: Turning Standards and Resilience into Outcomes
If you work within the advanced therapy ecosystem, you already know the story of 2025 from an industry standpoint, where science continued accelerating while funding and commercialization hinged on whether the supporting systems could keep up. For Cryoport Systems, that reality was a catalyst. This year, we raised the bar yet again and demonstrated how having the right strategic partnership behind your end-to-end supply chain can turn logistics into leverage, can transform compliance into confidence, and can spin scale into speed.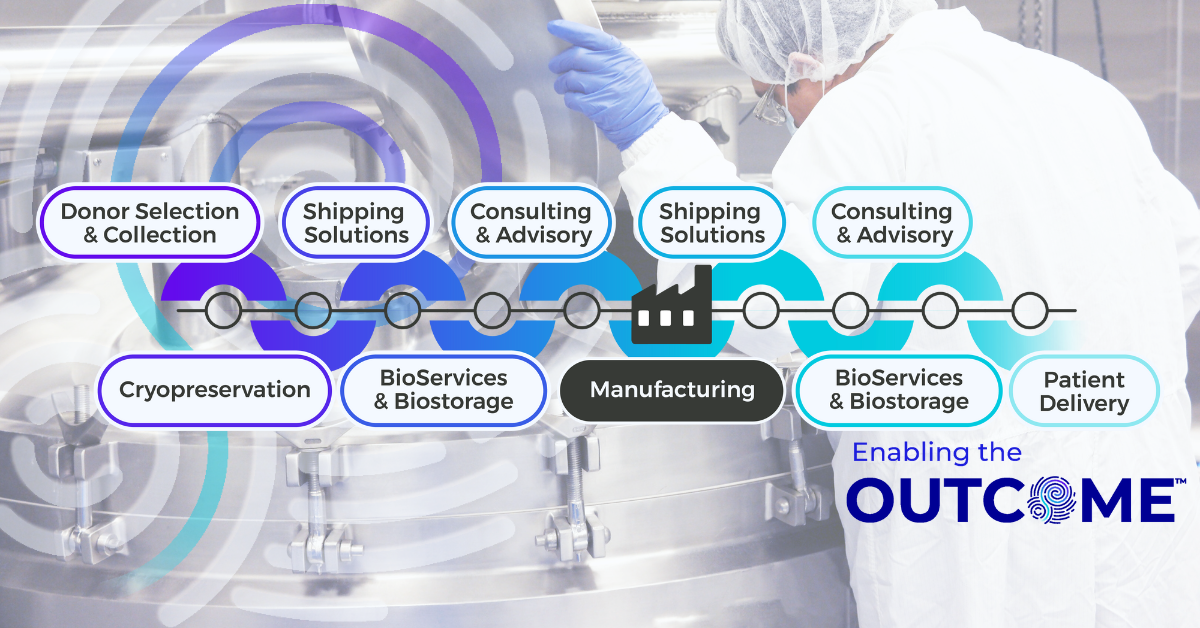
10/15/2025
The Connected Chain: Why Supply Chain Integration Matters in Advanced Therapy Manufacturing
Contract Development and Manufacturing Organizations (CDMOs) are at the heart of the advanced therapy ecosystem, managing complex, high-stakes programs that demand speed and compliance. As therapies move from early development into clinical trials and through to commercialization, every stage of manufacturing, packaging, biostorage, and final drug delivery need to align perfectly for seamless execution. Yet in many cases, these functions are managed by multiple vendors, each operating in isolation. The result is a fragmented supply chain that increases risk while slowing execution due to layers of operational burden.
09/10/2025
Scale Smarter: How Global Supply Chain Centers Amplify CGT Program Growth
Cell and gene therapy (CGT) programs are among the most complex and sensitive operations in the life sciences. From cryopreservation and biostorage to logistics and regulatory compliance, every step in the supply chain can influence product integrity, clinical timelines, and the scalability of the program. For program heads as well as clinical and technical operations teams, the challenge becomes bigger than merely moving materials. It quickly becomes a challenge of scaling efficiently while maintaining compliance and supporting growth across a global network.
08/20/2025
Protecting the Foundation of Biologics with Flexible, Scalable Cell Banking
For any biotherapeutic program, your cell bank is the foundation of your manufacturing process. Master cell banks (MCBs), working cell banks (WCBs), and research cell banks (RCBs), represent years of development work and intellectual property. They are irreplaceable assets, and the security of these assets is directly tied to the long-term success of your program.
06/19/2025
Supporting Access Through Supply Chain Excellence on World Sickle Cell Day
World Blood Donor Day serves as a reminder of the life-saving impact of voluntary blood and cell donations. These donations, often behind the scenes and not always recognized, are essential to emergency and surgical medicine and increasingly play an important role in cell and gene therapies. At Cryoport Systems, we recognize the incredible value of donors. We also understand that moving these donations safely, reliably, and under the strictest conditions, is a critical link between compassion and cure.
05/29/2025
Starting Smart: How to Structure an Integrated Supply Chain in Early-Stage CGT Programs
For many academic labs, technology spinouts, and early-stage CGT companies, the temperature-controlled supply chain feels like something to figure out later. But when dealing with highly sensitive starting materials and therapies like CGTs, later can quickly become too late. Whether you’re preparing for first-in-human trials or just beginning to plan for scale, integrated thinking now can spare you time, cost, and complexity later.
05/19/2025
Clinical Trials Day: Supporting the Journey from Discovery to Delivery
Clinical trials represent hope, innovation, and the pursuit of better outcomes. They are the backbone of developing new diagnostics, vaccines, devices, and therapies. They help ensure safety, efficacy, and accessibility to the people who need them. That's why each year on May 20, the community comes together to celebrate Clinical Trials Day, honoring the anniversary of the first randomized clinical trial. At Cryoport Systems, we are honored to support our partners and clients in this journey.Categories
- All (61)
- Industry Insights (20)
- Managing the Cold Chain (48)
- Navigating Logistics (27)
- Patient Access and Awareness (8)
Tags
- Asia
- ATMPs
- Biologics
- BioServices
- Biostorage
- CDMO
- CDMOs
- Cell Therapy
- Clinical Trials
- Cold Chain Management
- Commercialization
- CROs
- Cryopreservation
- Europe
- Fragmentation
- Gene Therapy
- Global Logistics Tracking
- Global Supply Chain Management
- International Shipping
- North America
- Risk Mitigation
- Standards and Compliance
- Supply Chain Management