Supply Chain Archives

01/27/2026
Establish Stability from the Start with Frozen Starting Materials for Cell Therapy Development
For many years, cell therapy programs defaulted to fresh leukapheresis-derived starting material. The assumption was that if you could minimize the time from collection to manufacturing, you would maximize viability. As a result, fresh apheresis became not only a scientific preference but an operational tradition, shaping how clinical teams would schedule donors, how manufacturing suites would allocate capacity, and how programs would structure day-to-day execution. Program leaders treated any deviation from “fresh” as a risk to be justified rather than a choice to be evaluated. But reality keeps getting in the way.
01/27/2026
How Defragmenting the Pre-Clinical Supply Chain De-Risks Downstream Operations
Every stage of the supply chain including cryopreservation, logistics, and BioServices and biostorage, must align to ensure a seamless transition from pre-clinical work into Phase I trials. However, early-stage teams often select multiple vendors who each handle one piece of the process because it feels faster or more cost-effective in the moment. This approach, however, builds fragmented supply chains that introduce gaps as programs scale. And when those gaps surface, teams must devote valuable time and effort simply to get back on track.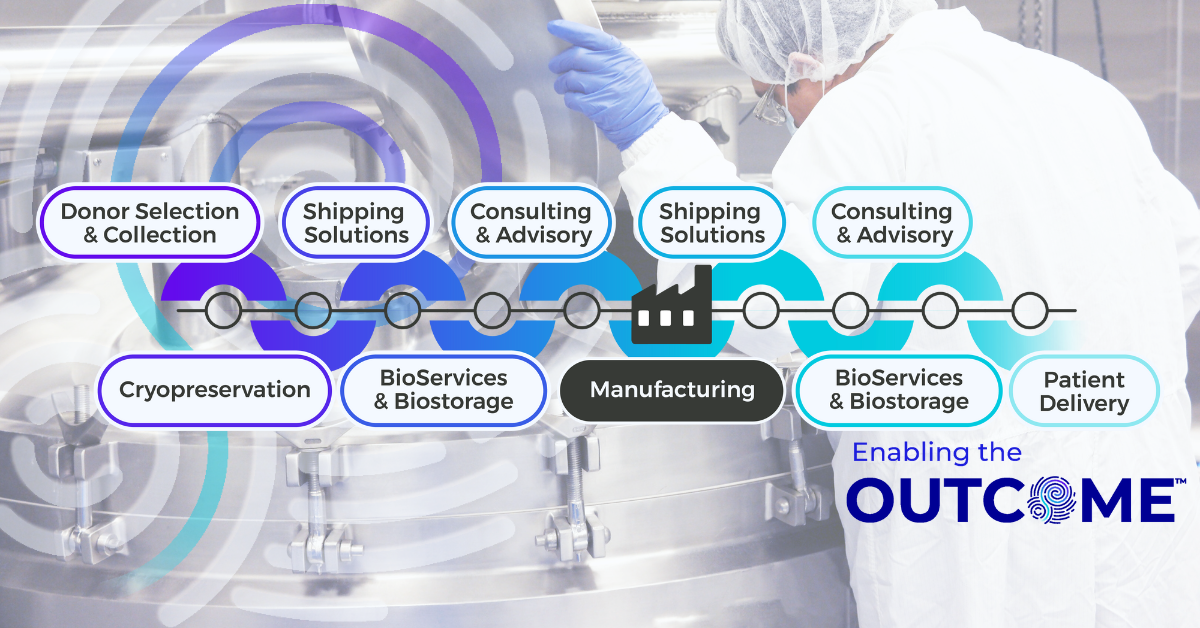
10/15/2025
The Connected Chain: Why Supply Chain Integration Matters in Advanced Therapy Manufacturing
Contract Development and Manufacturing Organizations (CDMOs) are at the heart of the advanced therapy ecosystem, managing complex, high-stakes programs that demand speed and compliance. As therapies move from early development into clinical trials and through to commercialization, every stage of manufacturing, packaging, biostorage, and final drug delivery need to align perfectly for seamless execution. Yet in many cases, these functions are managed by multiple vendors, each operating in isolation. The result is a fragmented supply chain that increases risk while slowing execution due to layers of operational burden.
10/01/2025
Cryoport Systems Redefines the Global Supply Chain with Launch of New Facility Near Paris, France
Cryoport Systems is thrilled to announce the grand opening of our revolutionary Global Supply Chain Center in Louvre, France. This cutting-edge campus is a one-stop location for end-to-end, temperature-controlled supply chain solutions and is designed to tackle the most complex challenges in the life sciences industry.
09/10/2025
Scale Smarter: How Global Supply Chain Centers Amplify CGT Program Growth
Cell and gene therapy (CGT) programs are among the most complex and sensitive operations in the life sciences. From cryopreservation and biostorage to logistics and regulatory compliance, every step in the supply chain can influence product integrity, clinical timelines, and the scalability of the program. For program heads as well as clinical and technical operations teams, the challenge becomes bigger than merely moving materials. It quickly becomes a challenge of scaling efficiently while maintaining compliance and supporting growth across a global network.
08/20/2025
Protecting the Foundation of Biologics with Flexible, Scalable Cell Banking
For any biotherapeutic program, your cell bank is the foundation of your manufacturing process. Master cell banks (MCBs), working cell banks (WCBs), and research cell banks (RCBs), represent years of development work and intellectual property. They are irreplaceable assets, and the security of these assets is directly tied to the long-term success of your program.
07/25/2025
Scaling with Confidence for CDMO Supply Chain Alignment
Contract Development and Manufacturing Organizations (CDMOs) play a critical role in the life sciences industry, enabling biopharma innovators to bring new therapies from development to manufacturing to patients around the world. The demand placed on CDMOs has only increased in recent years, especially as cell, gene, and biologic therapies have moved from concept to clinic at unprecedented speeds. While the technical and scientific capabilities of CDMOs have advanced to meet these challenges, one area remains a frequent source of friction and delay...the end-to-end, temperature-controlled supply chain.
06/24/2025
When Every Hour Counts: How Cryoport Systems Rapidly Mobilized to Protect INmune Bio’s Critical Clinical Materials
Biopharmaceutical development, particularly for advanced CGTs, relies on a robust infrastructure of logistics, BioServices, and temperature-controlled supply chains to ensure timely and intact delivery of drug products under stringent regulations. INmune Bio Inc. faced a challenge when a sudden biostorage site closure jeopardized a key clinical trial. However, with a strong support network, they turned to established partners like Cryoport Systems to overcome the crisis.
06/14/2025
Recognizing Donors and Advancing the Future of Cell Therapy
World Blood Donor Day serves as a reminder of the life-saving impact of voluntary blood and cell donations. These donations, often behind the scenes and not always recognized, are essential to emergency and surgical medicine and increasingly play an important role in cell and gene therapies. At Cryoport Systems, we recognize the incredible value of donors. We also understand that moving these donations safely, reliably, and under the strictest conditions, is a critical link between compassion and cure.
03/21/2025
Strengthening ATMP Development in Europe: How BioServices Centers in France Enable Efficiency and Scalability
As Advanced Therapy Medicinal Product (ATMP) developers progress from early-stage research to late-phase clinical trials and commercialization, biostorage, sample management, and regulatory-compliant logistics become increasingly complex. Efficiently managing these critical components is essential to ensure product integrity, regulatory compliance, and seamless supply chain operations.Categories
- All (61)
- Industry Insights (20)
- Managing the Cold Chain (48)
- Navigating Logistics (27)
- Patient Access and Awareness (8)
Tags
- Asia
- ATMPs
- Biologics
- BioServices
- Biostorage
- CDMO
- CDMOs
- Cell Therapy
- Clinical Trials
- Cold Chain Management
- Commercialization
- CROs
- Cryopreservation
- Europe
- Fragmentation
- Gene Therapy
- Global Logistics Tracking
- Global Supply Chain Management
- International Shipping
- North America
- Risk Mitigation
- Standards and Compliance
- Supply Chain Management