Integrated Global Supply Chain Centers

Specialized storage and distribution solutions for your evolving needs.
Manufacturing scalability is a prominent challenge within the life sciences industry. The establishment of costly facilities, fluctuating manufacturing capacities, and shortage of specialized talent limit growth potential. For organizations to scale their operations, rigorous expansion within supply chain management services is needed to combat manufacturing limitations.
Cryoport Systems’ integrated global supply chain network is a first-of-its-kind system of connected services for the life sciences supply chain. Specifically designed in response to industry manufacturing needs, this network encompasses our logistics services and specialized storage and distribution solutions to support developers’ rapidly growing service requirements through our BioServices solutions.

Specialized Network of Solutions
Cryoport Systems’ BioServices solutions extend support to manufacturing operations to help combat scalability and production limitations. Our BioServices solutions encompass the following capabilities.
- GMP storage
- Biobanking
- Kit production
- Packaging & labeling
- Clinical sample management
- EU regulatory & QP services
- Drug return & destruction
These services coupled with our logistics expertise are the foundation for our network of supply chain solutions that allow Cryoport Systems to support our clients’ evolving storage and distribution needs throughout all stages of their manufacturing journey.

Global Supply Chain Centers
Our network of capabilities can be conducted at our Integrated Global Supply Chain Centers currently in Houston, Texas and Morris Plains, New Jersey within the United States. These unique centers manage all logistics, BioServices solutions, and specialized service operations within a single facility, providing our clients with the infrastructure and resources required to scale their operations. Once a client’s commodity arrives at an Integrated Global Supply Chain Center, it is then regulated through our advanced inventory management system that tracks a commodity’s:
- Chain of Custody
- Chain of Condition
- Chain of Identity
- Chain of Compliance®
This management system is an exclusive capability of our Integrated Global Supply Chain Centers and is not performed by our competitors. Our network of solutions conducted at our Integrated Global Supply Chain Centers makes Cryoport Systems the exclusive provider of scalable services for the life sciences from initial testing to commercialization.

The Future of Global Supply Chain Management
Our centers currently operating in Texas and New Jersey are only the start of our goal to build an expansive network of Integrated Global Supply Chain Centers. Cryoport Systems is already planning for more Integrated Global Supply Chain Centers in Southern California, France, the United Kingdom, and the Netherlands with others to follow. We will expand our global supply chain network on a continual process driven by client demand and industry needs.
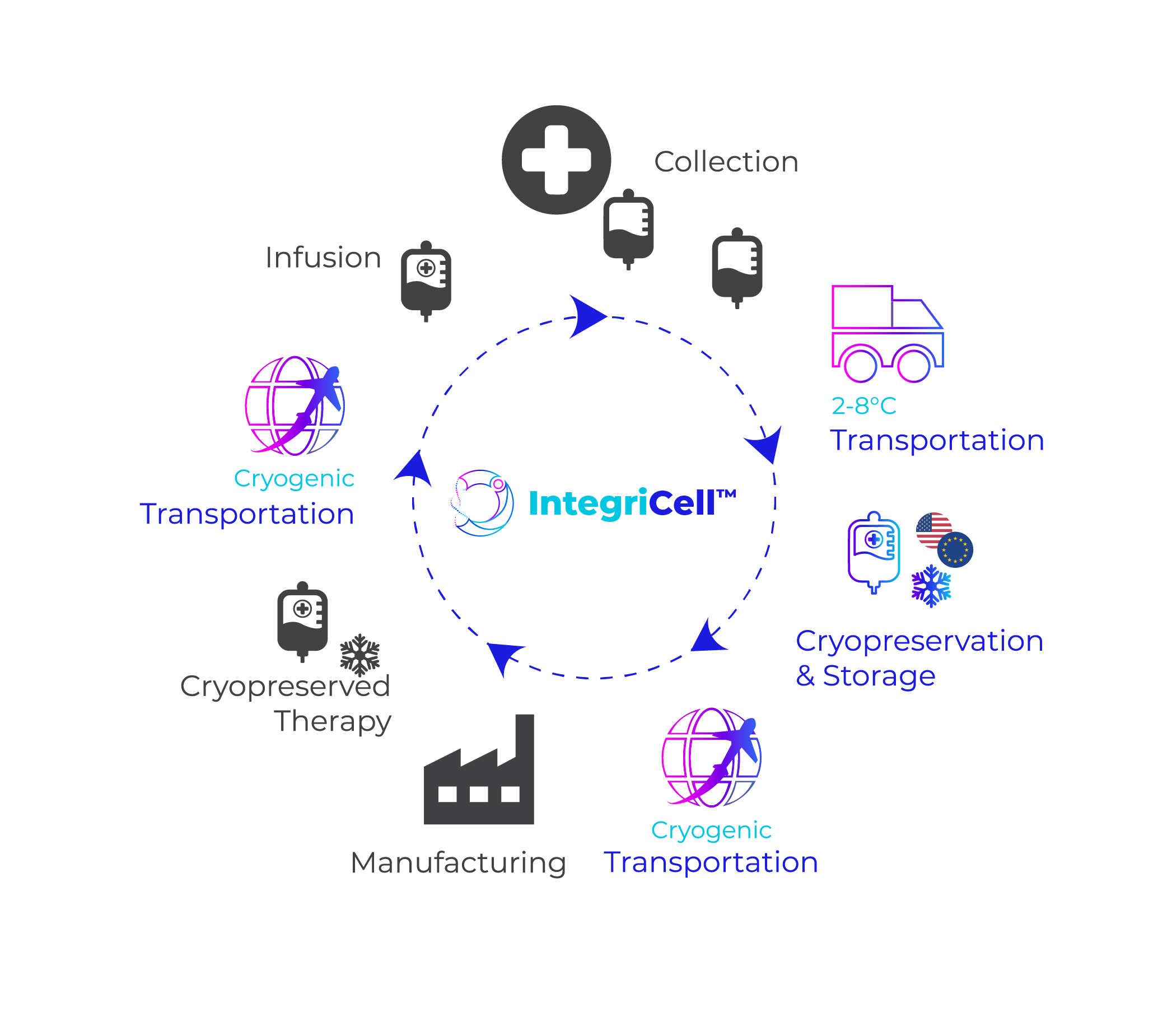
Our future facilities will also incorporate new technologies, automation, and streamlined operational processes. These advanced services will include our standardized cryopreservation and distribution solution for the global cell therapy market, IntegriCell™. As the life sciences industry continues to grow and change, the continual development of these Integrated Global Supply Chain Centers will enable Cryoport Systems to match the capacity and solutions required to support the biopharmaceutical, reproductive medicine, and animal health markets.