Supply Chain Archives
10/15/2025
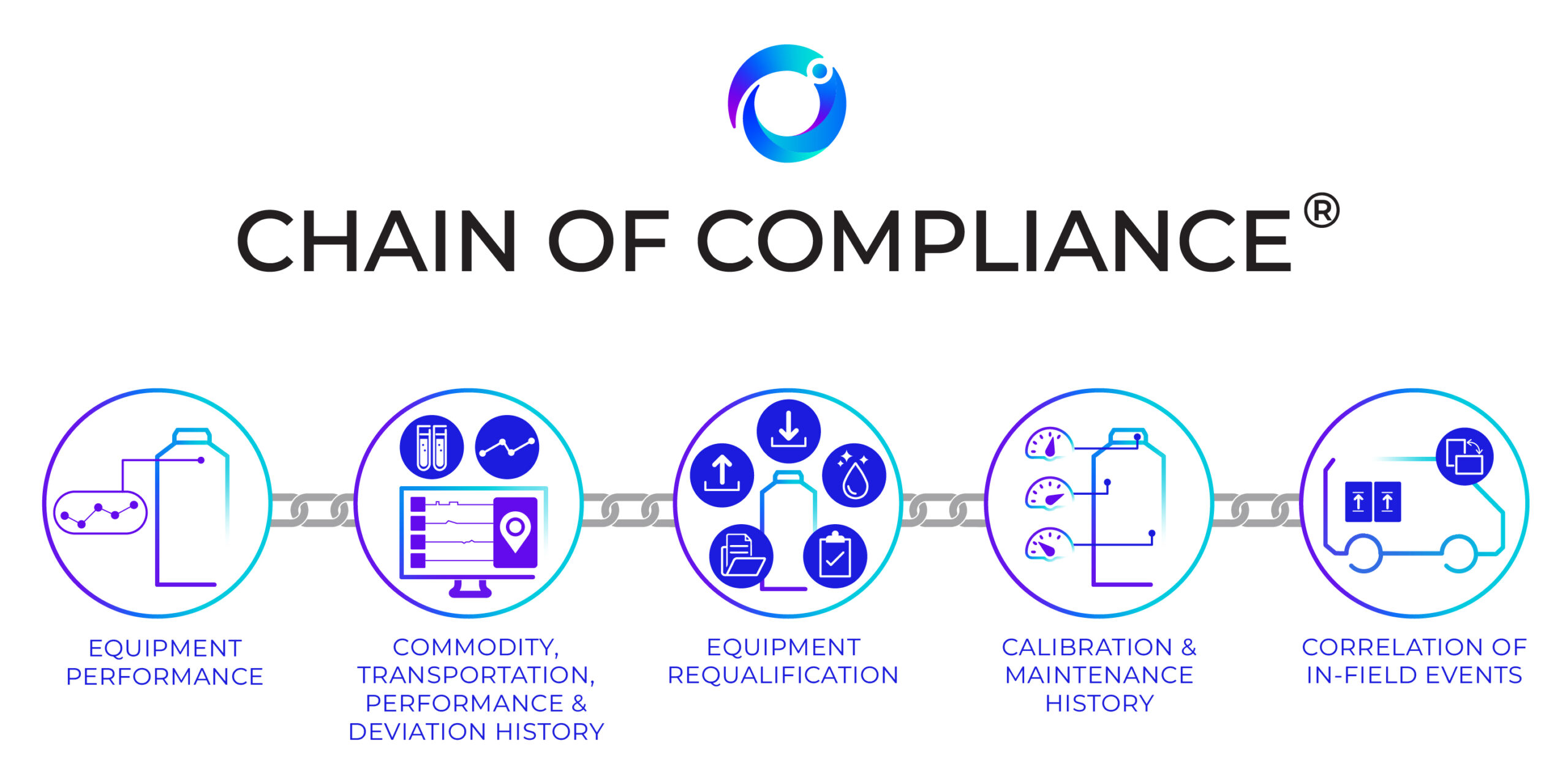
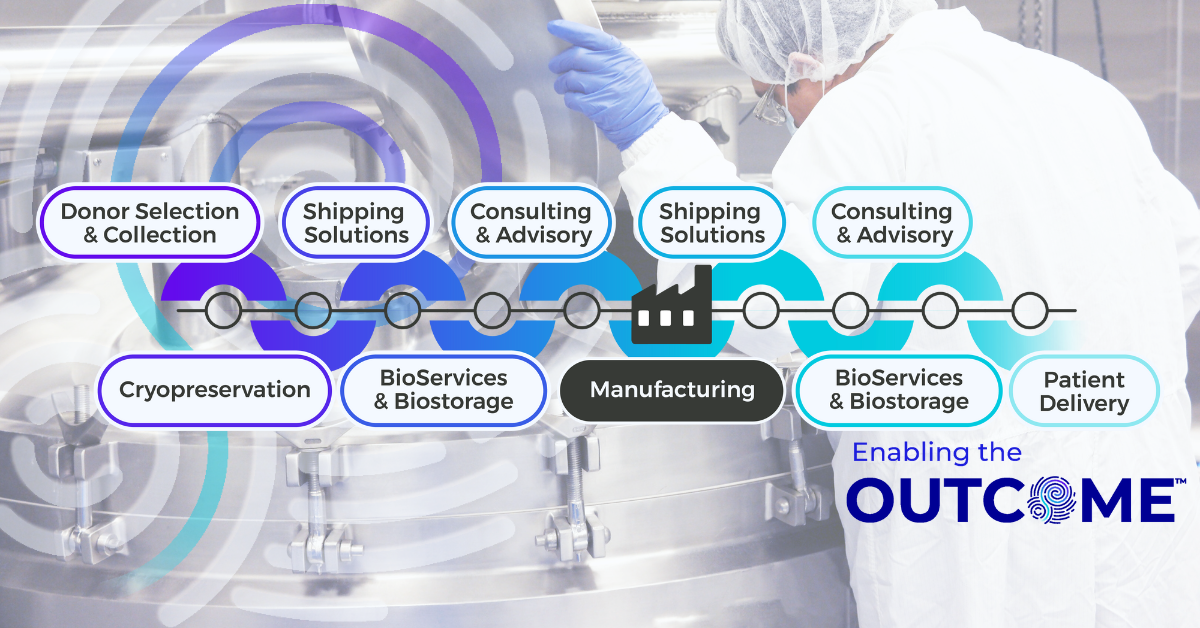
The Connected Chain: Why Supply Chain Integration Matters in Advanced Therapy Manufacturing
Contract Development and Manufacturing Organizations (CDMOs) are at the heart of the advanced therapy ecosystem, managing complex, high-stakes programs that demand speed and compliance. As therapies move from early development into clinical trials and through to commercialization, every stage of manufacturing, packaging, biostorage, and final drug delivery need to align perfectly for seamless execution. Yet in many cases, these functions are managed by multiple vendors, each operating in isolation. The result is a fragmented supply chain that increases risk while slowing execution due to layers of operational burden.
08/01/2025
Built for Speed: How Cryoport Systems Delivers Without Compromise
In the life sciences, time isn’t just money, it impacts patient outcomes, regulatory compliance, and even trial integrity. When a temperature-sensitive shipment is delayed due to inventory shipper shortages or logistical bottlenecks, the impact can cascade across an entire program. Unfortunately, these scenarios aren’t theoretical. When you’re working with irreplaceable biologic materials, this kind of disruption quickly moves from inconvenient to unacceptable.
03/24/2025
Strengthening Global ATMP Logistics: How Cryoport Systems’ Netherlands Facility Supports Temperature-Controlled Supply Chains
The success of ATMPs depends on more than just scientific breakthroughs, it requires a highly controlled, risk-mitigated supply chain that ensures product integrity from research through commercialization. With cell and gene therapies moving across multiple clinical sites, manufacturing facilities, and regulatory jurisdictions, developers need a logistics partner that can support seamless, temperature-controlled supply chain management at a global scale.
03/12/2025
The Gateway to Europe: How Cryoport Systems’ Stevenage Supply Chain Hub Supports ATMP Development
For UK-based Advanced Therapy Medicinal Product (ATMP) developers, access to European clinical trial sites and commercial markets is a critical component of a successful growth strategy. Yet, navigating regulatory barriers, customs challenges, and supply chain complexities can create unnecessary delays, increase costs, and put the integrity of sensitive biologics at risk.
12/30/2024
Supporting Compliance and Reducing Risk in Gene Therapy Logistics
When it comes to high stakes logistics in the gene therapy sector, adherence to regulatory standards isn’t just a box to check—it’s essential to delivering therapies that maintain integrity from production to patient. Cryoport Systems understands the unique risks in this space and has built compliance and risk mitigation into the very design of the Cryoport Elite™ Ultra Cold shipping system.
10/04/2024
Certify Safety for Human-derived Materials with Advanced Therapy Shippers®
The risk of contamination can compromise life-saving therapies, making it a major concern within the world of transporting precision medicines.
09/03/2024
Cryoshuttle® Service Brings Local Support to Stevenage, England
In June, Cryoport Systems officially launched our newest Global Supply Chain Hub in Stevenage, England, a renowned center for cell and gene therapy development within the U.K.
05/20/2024
Cryoport Elite® Shipping System: Industry-leading Advanced Therapy Support
The life sciences industry requires a robust shipping system that can handle the risks associated with transporting high-value commodities such as advanced therapies.
03/27/2024
Tec4med: The Newest Addition to Cryoport’s Team
Cryoport Systems is a part of a nexus of supply chain solutions companies all under Cryoport, Inc. The other business units are CRYOGENE, CRYOPDP, and MVE Biological Solutions...Categories
- All (61)
- Industry Insights (20)
- Managing the Cold Chain (48)
- Navigating Logistics (27)
- Patient Access and Awareness (8)
Tags
- Asia
- ATMPs
- Biologics
- BioServices
- Biostorage
- CDMO
- CDMOs
- Cell Therapy
- Clinical Trials
- Cold Chain Management
- Commercialization
- CROs
- Cryopreservation
- Europe
- Fragmentation
- Gene Therapy
- Global Logistics Tracking
- Global Supply Chain Management
- International Shipping
- North America
- Risk Mitigation
- Standards and Compliance
- Supply Chain Management