Cryoport’s Chain of Compliance®: Collects, Interprets, and Leverages Comprehensive Data to Enable a Significantly Smarter Supply Chain
Companies vying to be the first to market with breakthrough treatments have a great deal riding on the success of their efforts. Incorporating innovative processes, ethical considerations, assuring the safety and efficacy of the product, and managing the commodity’s environmental control are formidable challenges that often lack standardized quality control procedures. Precision medicine requires end-to-end traceability: everything from chain of custody, to chain of condition, to chain of identity. The fragility of regenerative medicine therapies and the need to manage risk means a fourth link in the chain will soon be a regulatory requirement.
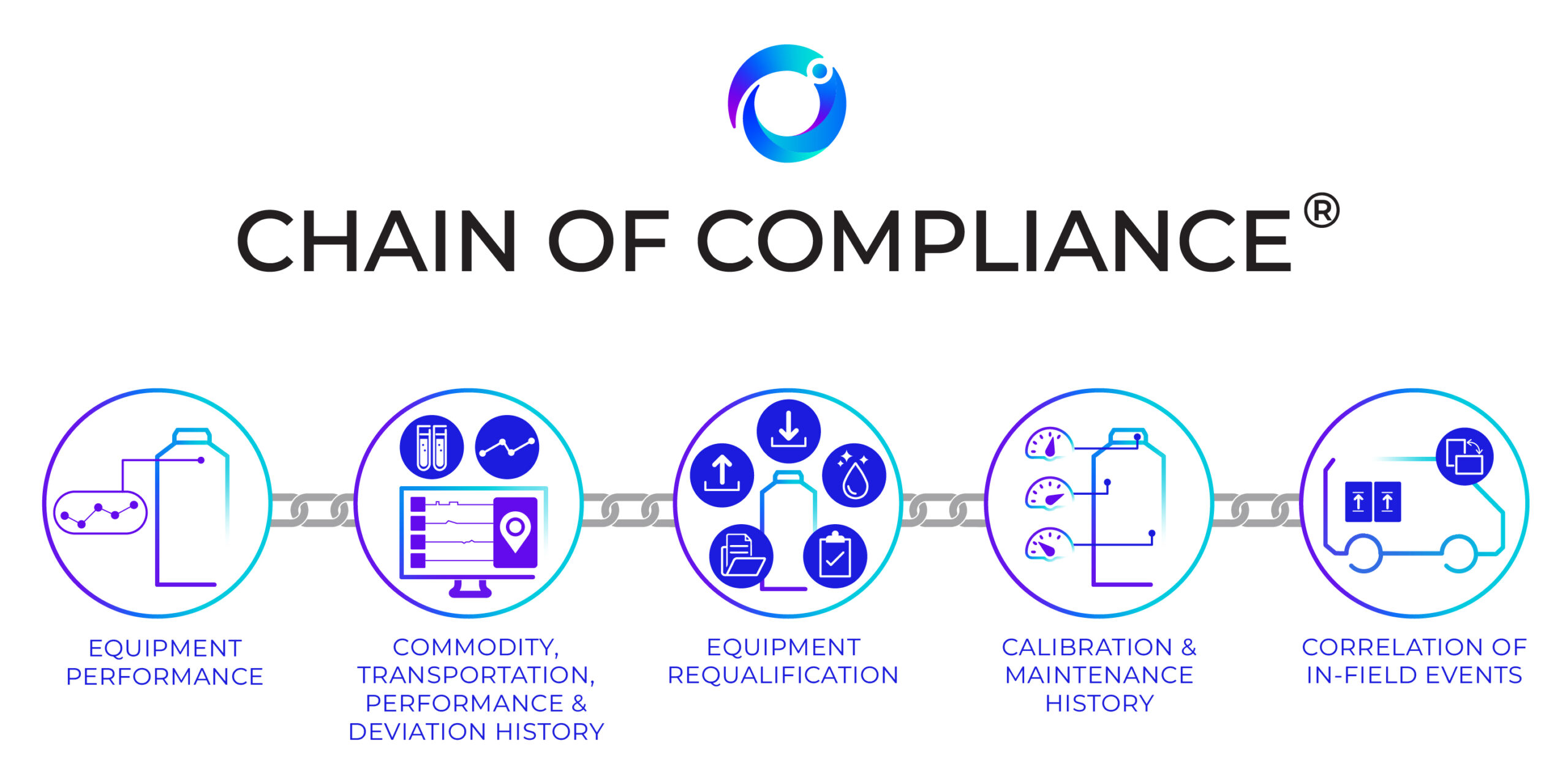
As a response to the industry’s demand to properly manage the sensitive aspects of drug products and the disproportionate risk of improper cold chain management, Cryoport developed a standard for distribution, Chain of Compliance®, to establish full traceability of all processes, data, and equipment used in managing the distribution of irreplaceable commodities. Compliance standards evaluate equipment performance, commodity history, equipment requalification, calibration history, and the correlation of in-field events and the impact on commodities.
Cryoport is the only logistics provider in the life science industry that can provide such traceability for the Regenerative Medicine space. Chain of Compliance® provides the ability to collect, interpret, and leverage comprehensive data enabling a significantly more intelligent supply chain. Cryoport provides equipment, processes and systems that support the Chain of Compliance® Standards:
- Cryoport Express® shippers are requalified after each use for cleanliness, LN2 capacity, and minimum required hold time threshold.
- Smartpak II® condition monitoring system is configured to provide visibility of both the conditions and location of your valuable sample throughout the entire shipment process and provide you with the data to prove that your sample integrity was maintained during transit.
- The Cryoportal® Logistics Management System is responsible for recording and maintaining the traceability of all the equipment, commodities, and in-field incidents, identifying where your commodity is and how it’s been treated — from loading, through storage and fulfillment, to delivery at its destination site.
- Veri-Clean® is the life science logistics industry’s first and only validated cleaning and disinfection process, reducing external contaminants by 99.9999%, virtually eliminating the risk of cross-contamination.
Robust data management is the key to mitigating risk and better understanding variabilities of an uncontrolled system. The ability to assess risk and provide intervention capabilities when unexpected problems arise will not only save you time and money, but also can save lives.
Contact the Cryoport team to learn more about the intricate details of each product and service that has been carefully developed to complete the Chain of Compliance®.


