Cell Therapy

Enabling the Outcome™ for Cell Therapy
Our expansive supply chain platform encompasses a full range of solutions
Cryoport Systems was purpose-built to support the life sciences. Today, our partnership capabilities have grown to include not only our expert temperature-controlled supply chain logistics, but to encompass a robust platform of supply chain solutions that support every stage and phase of cell therapy research, development, and manufacturing. With services that encompass BioServices and biostorage, cryopreservation, and custom-tailored consulting and advisory services in addition to our industry-leading shipping and logistics services, our support scales alongside your cell therapy development.
End-to-End Support Built Around Your Cell Therapy.
From donor collection through patient delivery, Cryoport Systems delivers a fully integrated supply chain platform that simplifies coordination, reduces risk, and keeps cell therapies moving forward.
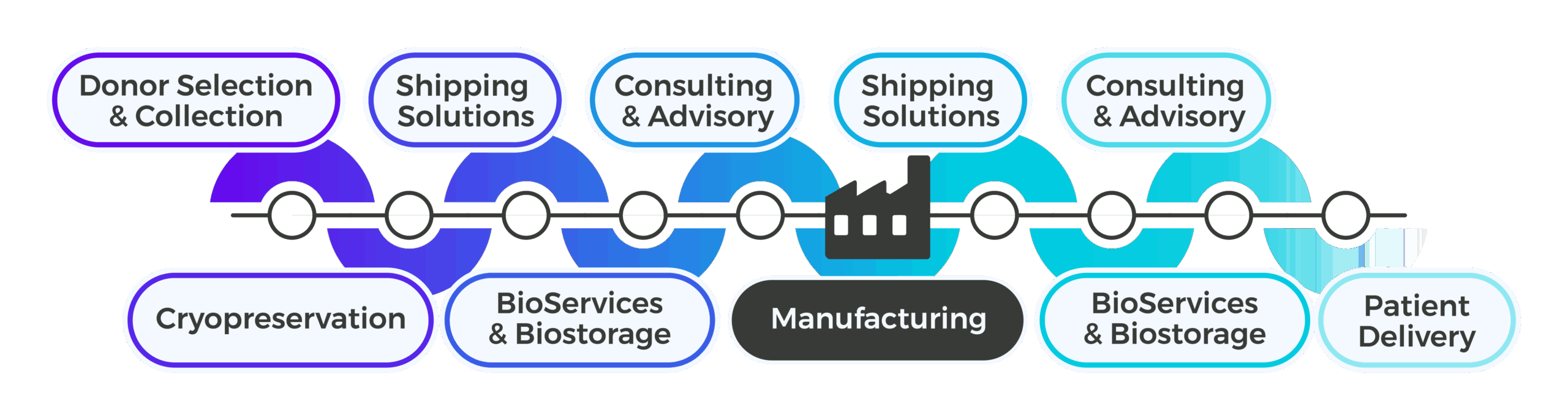
Support Upstream of Manufacturing
Upstream of manufacturing, Cryoport Systems delivers the standardization and control cell therapy programs need to manage donor- and patient-derived materials with precision. With our modular, customizable, end-to-end platform approach, we can produce and deliver fully standardized apheresis collection kits and pre-conditioned shipping systems to collection sites, ensuring consistency and compliance from the point of collection. We offer IntegriCell™ cryopreservation, which uses an automated closed process (or your own protocol) that is optimized to preserve cellular viability and reduce variability. With secure biostorage options and just-in-time delivery to manufacturing, plus early-phase consulting and advisory services for risk assessments, shipping lane qualifications, and shipper and packaging performance qualifications, we help de-risk critical upstream workflows and eliminate fragmentation before manufacturing even begins.
Support Downstream of Manufacturing
Downstream of manufacturing, Cryoport Systems supports the highly personalized demands of the cell therapy supply chain with an integrated, global infrastructure. Our validated shipping systems protect sensitive final products as they move toward clinical sites or patients, while advanced BioServices streamline operations with pick, pack, label, and distribution tailored to therapy-specific requirements. As programs expand, we provide consulting and advisory services to qualify new shipping lanes, support geoexpansion, and manage QP release across the EU. Final mile logistics are handled with the same precision and Chain of Compliance®, ensuring patient-ready doses arrive safely and on time. With Cryoport Systems as a single vendor partner, cell therapy developers avoid delays, reduce risk, and maintain full control across every downstream step.
Support for CDMOs
Cryoport Systems supports CDMOs engaged in cell therapy development and manufacturing, with the full understanding that variability, tight timelines, and material fragility requires supply chain precision. From early-phase clinical trials to commercial launch, our fully integrated platform enables CDMOs to manage every movement of cellular materials with confidence. Whether handling autologous or allogenic starting material or looking for specialized biostorage for master cell banks, our packaging, logistics, and BioServices and biostorage solutions ensure traceability, temperature control, and full regulatory adherence at every touchpoint. Our team brings hands-on knowledge of cell therapy manufacturing workflows, allowing us to anticipate logistical bottlenecks and proactively implement safeguards that protect product integrity from vein to vein.
Cryoport Systems is the single-vendor platform trusted by leading CDMOs to reduce variability, enable traceability, and maintain cell viability across the global cell therapy supply chain.
We support CDMOs with highly customized services, from clinical sample management and Qualified Person (QP) oversight for European imports to validated cryogenic shipping systems engineered specifically for living cells. Our Chain of Compliance® tracks the condition and identity of each shipment continuously, enabling regulatory-grade documentation and full chain of custody visibility for audit support. We offer proactive risk mitigation with our lane qualification and consulting and advisory expertise, tailored to the unique timelines and variability of cell therapy programs. As CDMOs take on increasingly complex therapeutic development and manufacturing, Cryoport Systems helps ensure that your end-to-end supply chain is managed to the same rigorous standard of care your clients expect.
Support for CROs
Cell therapy programs require a new standard when it comes to the orchestration of living materials, chain of custody compliance, and milestone adherence, especially when trial timelines and patient availability are non-negotiable. Cryoport Systems enables CROs to operate as an extension of the sponsor, with a fully integrated logistics and BioServices platform that spans cryopreservation, biostorage, consulting and advisory support, and logistics and transportation. With over 700 clinical trials and 18 commercial advanced therapies supported to date, we bring the experience, scale, and standardization CROs need to confidently manage even the most complex cell therapy protocols.
We equip CROs to deliver milestone-driven cell therapy trials with traceable, risk-mitigated solutions that prioritize patient safety and regulatory excellence.
Our proprietary Chain of Compliance® framework ensures that every handoff from collection through final patient delivery is fully traceable and aligned with GMP/GDP standards. We offer dedicated consulting and advisory support for early risk assessments, shipping lane qualifications, and planning for supporting documentation for regulatory filings, and our IntegriCell™ cryopreservation experts enable seamless transitions of manufacture-ready cryopreserved leukopaks between clinical sites and manufacturing with minimal viability loss and extended manufacturability timelines. With regional centers in the US, EU, and APAC, CROs gain consistent, standardized support and localized expertise, delivering global reach without vendor fragmentation. When timelines are tight and regulatory oversight is high, we make it easier for CROs to protect materials and meet every milestone with confidence.
Support for Therapeutic Developers
Cell therapy sponsors operate in one of the most complex segments of the life sciences industry, where the product is often personalized, time-sensitive, and irreplaceable. Cryoport Systems supports therapeutic developers with a deeply integrated supply chain platform that protects patient-specific material from collection through final administration. Whether you’re managing autologous collections or scaling allogeneic therapies for broader clinical deployment, we provide the infrastructure needed to maintain viability and compliance at every stage. From early clinical development through commercial launch, we help developers reduce risk and keep therapies on track.
Cryoport Systems delivers the logistical precision and operational resilience cell therapy developers need to scale confidently and protect every patient’s outcome.
Our end-to-end model unifies validated shipping systems, standardized collection kits, centralized cryopreservation via IntegriCell™, and purpose-built biostorage, all backed by expert consulting and advisory support that helps de-risk each phase of development. We provide Chain of Compliance® tracking to preserve chain of identity and condition across shipments, support regulatory filings with audit-ready documentation, and enable geographic expansion with global infrastructure and QP release services in the EU. By consolidating services under one partner, therapeutic developers eliminate supply chain fragmentation and gain a scalable foundation designed to uphold the highest standards of quality, safety, and care.
Support for Every Stage of Development.
Cryoport Systems provides a comprehensive range of integrated temperature-controlled supply chain solutions. Find your perfect solution by clicking through the dropdown below.
Select development phase:

Leverage a platform with a proven track record of compliance, reliability, and innovation.
- IntegriCell™ Cryopreservation
Consistent, high-quality leukapheresis starting material.
Learn More >> - BioServices & Biostorage
Integrated solutions to support advanced therapies.
Learn More >> - Consulting & Advisory Services
Our team of experts can help de-risk your logistics.
Learn More >> - Shipping Solutions
Temperature-controlled packaging solutions.
Learn More >> - Transportation
Transportation of commodities at various temperatures.
Learn More >>
Cryoport Systems is Enabling the Outcome™ through our unparalleled supply chain platform.
Offering a comprehensive scope of robust supply chain management solutions and trusted logistics.
Cryoport Systems was established by a team of doctors dedicated to serving the life sciences through expert logistics support. Today, our partnership capabilities have evolved beyond logistics into a robust platform of supply chain solutions that supports every stage and phase of the life sciences research, development, and manufacturing process. Our comprehensive platform of solutions makes Cryoport Systems a standalone vendor that can support all aspects of the temperature-controlled supply chain.
LEARN MORE ABOUT HOW CRYOPORT SYSTEMS IS ENABLING THE OUTCOME™