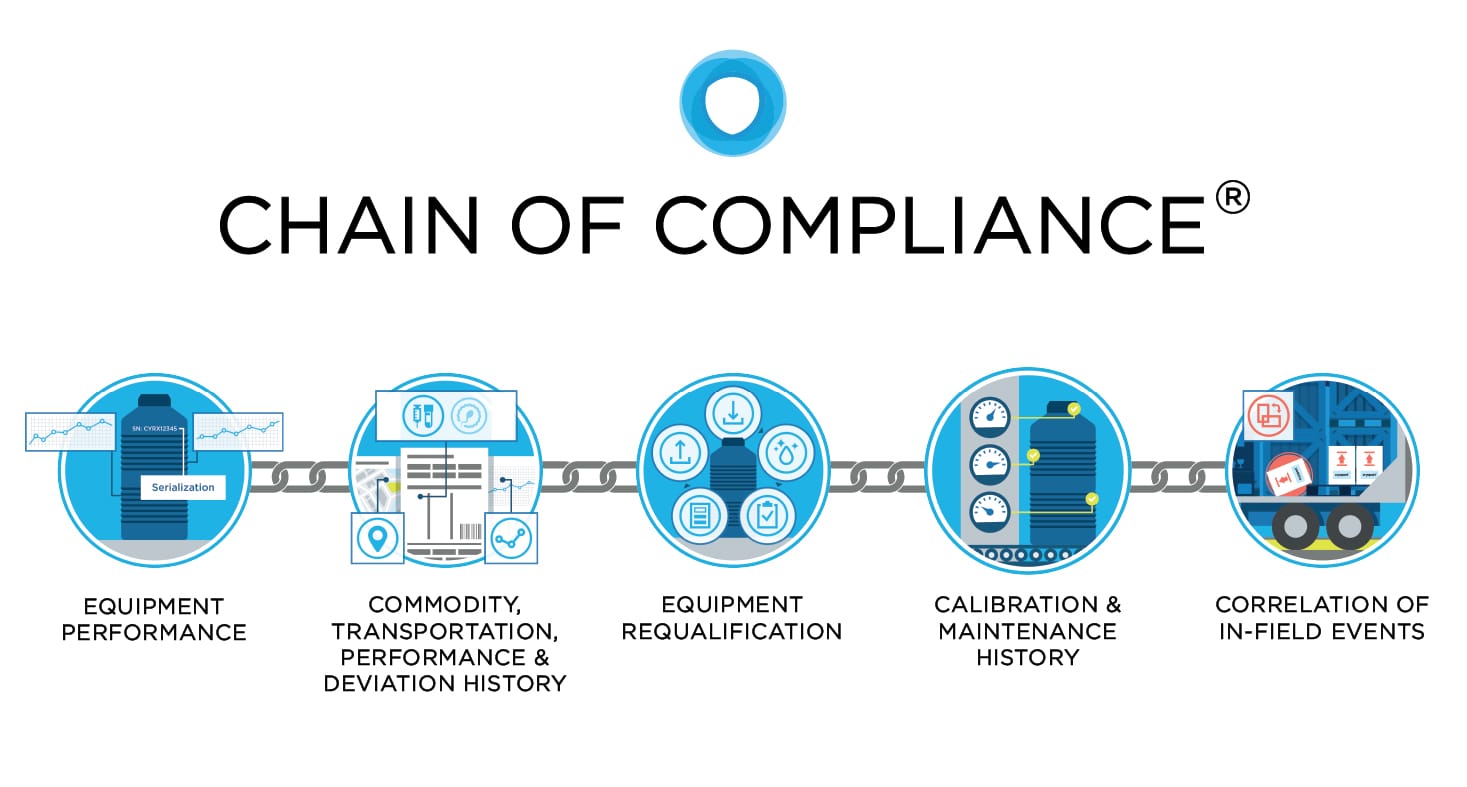
Cryoport’s Chain of Compliance®: Collects, Interprets, and Leverages Comprehensive Data to Enable a Significantly Smarter Supply Chain
Companies attempting to advance Reproductive Medicine have a great deal riding on the success of their efforts. Incorporating innovative processes, ethical considerations, assuring the safety and efficacy of the reproductive product, and managing the environment are formidable challenges that often lack standardized quality control procedures. Precise procedures require end-to-end traceability: everything from chain of custody to chain of condition, to chain of identity. The fragility of reproductive medicine and the need to manage risk means a fourth link in the chain will soon be a standardized quality process
As a response to the industry’s demand to properly manage the sensitive aspects of the fertility journey and the disproportionate risk of improper cold chain management, Cryoport developed a standard for distribution, Chain of Compliance®, to establish full traceability of all processes, data, and equipment used in managing the distribution of irreplaceable commodities. Compliance standards evaluate equipment performance, commodity history, equipment requalification, calibration history, and the correlation of in-field events and the impact on commodities.
Cryoport Express® shippers are requalified after each use for cleanliness, LN2 capacity, and minimum required hold time threshold.
The Cryoportal® logistics management system and the Smartpak™ condition monitoring system are responsible for recording and maintaining the traceability of all the equipment, commodities, and in-field incidents, identifying the location of your commodity and how it’s been treated—from loading, through storage and fulfillment, to delivery at its destination site.
Veri-Clean® is the life science logistics industry’s first and only validated cleaning and disinfection process, reducing external contaminants by 99.9999%, virtually eliminating the risk of cross-contamination.
Robust data management is the key to mitigating risk and better understanding variabilities of an uncontrolled system. The ability to assess risk and provide intervention capabilities when unexpected problems arise will not only save you time and money, but also can save lives.
Contact the Cryoport team to learn more about the intricate details of each product and service that has been carefully developed to complete the Chain of Compliance®.