The Connected Chain: Why Supply Chain Integration Matters in Advanced Therapy Manufacturing
Contract Development and Manufacturing Organizations (CDMOs) are at the heart of the advanced therapy ecosystem, managing complex, high-stakes programs that demand speed and compliance. As therapies move from early development into clinical trials and through to commercialization, every stage of manufacturing, packaging, biostorage, and final drug delivery need to align perfectly for seamless execution. Yet in many cases, these functions are managed by multiple vendors, each operating in isolation. The result is a fragmented supply chain that increases risk while slowing execution due to layers of operational burden.
Fragmentation may not be visible day-to-day, but it shows up when timelines tighten or an unexpected deviation from the plan occurs. When data lives in separate systems or when responsibilities are divided among multiple providers, the impact can be significant. Each handoff becomes a potential point of failure, and one delay leads to another. For CDMOs, that means less time spent on science and production and more time spent troubleshooting the mechanics of the supply chain.
To meet today’s demands and prepare for tomorrow, CDMOs need supply chains that are intentionally designed for seamless continuity, connecting every component and partner within a single, integrated framework.
When Complexity Creates Fragmentation
The advanced therapy supply chain is inherently complex. Cell and gene therapies (CGTs), viral vectors, and other advanced therapy medicinal products (ATMPs) require precise temperature control, rigorous chain-of-identity and chain-of-custody verification, and continuous oversight throughout the product journey to maintain product viability and regulatory compliance.
In a traditional multi-vendor model, each service is managed independently. Each has its own systems, SOPs, documentation standards, quality guidelines, and communication channels. On the surface, this may appear efficient – especially when each vendor is highly specialized. But when these moving parts are out of alignment, small inefficiencies can quickly scale into larger issues.
Communication breakdowns and misaligned quality standards, for example, can create hidden vulnerabilities. Every handoff becomes a point of exposure. For CDMOs managing multiple client programs, this fragmentation multiplies risk and complexity. Teams spend valuable time coordinating between suppliers and reconciling data, time that could otherwise be focused on production and client delivery.
Integration as a Foundation for Efficiency
An integrated supply chain changes the equation entirely. When critical activities upstream and downstream of manufacturing operate under a single, unified framework, the process becomes inherently more efficient. There’s no need to reconcile data across platforms, coordinate between various vendors, or manage redundant documentation for each patient dose. Communication flows directly, and decisions can be more easily made in real time.
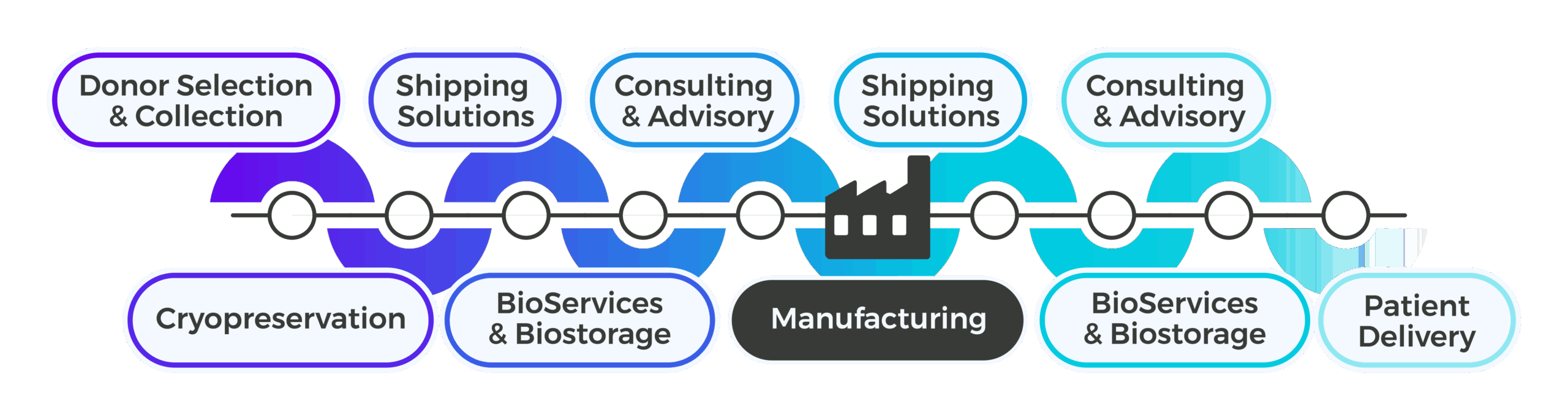
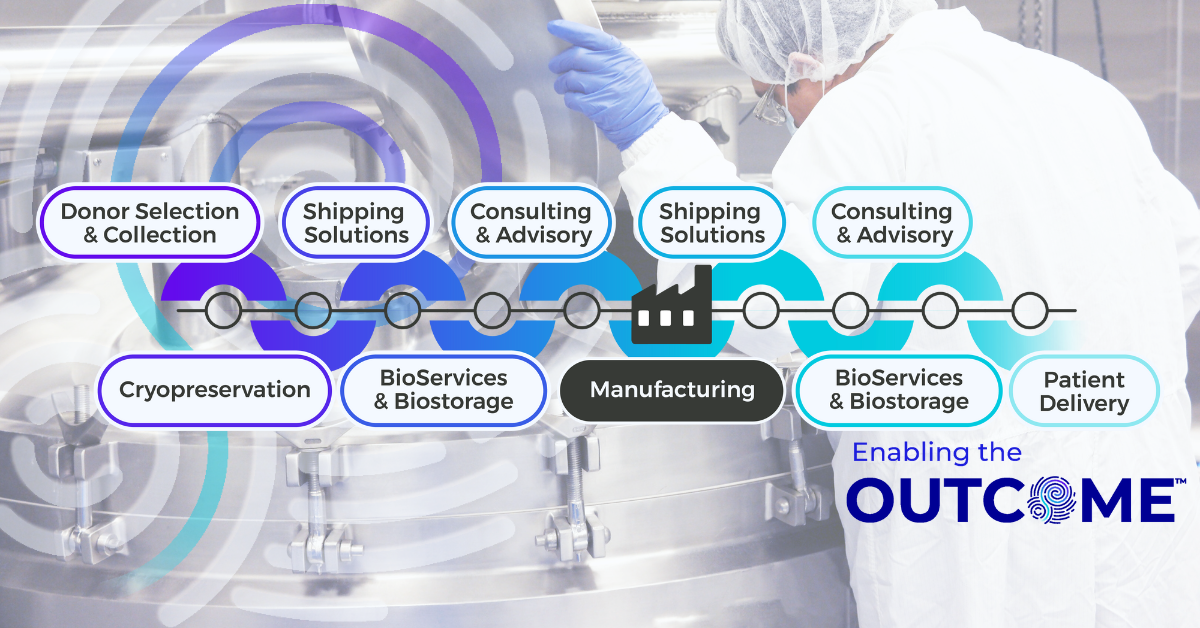
Upstream of manufacturing, Cryoport Systems delivers the standardization and control needed to manage donor- and patient-derived materials with precision. With our customizable end-to-end platform approach, we can produce and deliver standardized apheresis collection kits and pre-conditioned shipping systems to collection sites, ensuring consistency and compliance from the point of collection. We offer IntegriCell® cryopreservation services, which uses an automated closed process (or your own protocol) that is optimized to preserve cellular viability and reduce variability. With secure onsite biostorage options and just-in-time delivery to manufacturing, plus early-phase consulting and advisory services for risk assessments, shipping lane qualifications, and shipping system and packaging performance qualifications, we help de-risk critical upstream workflows and eliminate fragmentation before manufacturing even begins.
Downstream of manufacturing, we support the unique demands of the CGT supply chain with an integrated, global infrastructure. Our validated shipping systems protect sensitive final products as they move toward clinical sites or patients, while advanced BioServices streamline operations with pick, pack, label, and distribution operations tailored to therapy-specific requirements. As programs expand, we provide consulting and advisory services to qualify new shipping lanes, support geoexpansion, and manage qualified person (QP) release across the European Union. Final mile logistics are handled with the same precision and Chain of Compliance®, ensuring patient-ready doses arrive safely and on time.
For CDMOs, this means greater control and fewer points of failure. It reduces administrative burden, reduces capital expenditures, accelerates response times, and ensures that every handoff happens within a standardized, compliant process that supports global programs.
Efficiency in an integrated model is all about predictability. CDMOs can plan, execute, and scale with confidence because every link in the chain operates under the same quality management system, documentation standards, and escalation procedures. That consistency strengthens audit readiness and eliminates the delays that can occur when multiple suppliers need to align on processes and approvals.
Data Integration and Continuous Visibility
Beyond the structural aspects of an integrated supply chain are the digital requirements. A connected supply chain depends on unified data visibility that allows all stakeholders to monitor the movement and condition of materials in real time.
Cryoport Systems operates under our proprietary Chain of Compliance® standard that delivers exactly that. Each shipping system is linked to its complete performance and qualification history. This traceability ensures that every shipment can be tied back to a fully documented and verifiable chain of control.
The Cryoportal® logistics management system integrates environmental data such as temperature, orientation, humidity, and shock, providing continuous monitoring and visibility across the entire logistics network. This allows potential risks to be identified and ensures corrective actions can be taken immediately should any deviations occur, and provides CDMOs and their clients with a single, validated source of truth for every detail of every shipment.
Beyond compliance, this data integration enables insight. Over time, CDMOs gain performance trends that can inform critical workflows like process optimization, capacity planning, and even future program design. This allows an integrated supply chain model to not only improve day-to-day operations, but also to support longer-term strategic decision making.
The Power of a True Partnership
The difference between managing suppliers and partnering with an integrated provider goes beyond operational and instead becomes relational. A single-vendor model creates a partnership based on shared accountability and a mutual understanding of goals.
Cryoport Systems functions as an extension of CDMO operations, embedding within their quality and logistics frameworks rather than operating outside of them. This level of alignment allows for proactive collaboration, and when challenges arise, there are no delays in escalation or ambiguity in roles and responsibilities because both organizations are already working together and toward the same outcome.
For CDMOs, this partnership model streamlines everything from vendor management to client engagement. With one integrated partner responsible for the full end-to-end supply chain, efforts are streamlined and execution accelerates. This not only reduces administrative overhead, it also frees up operational bandwidth.
As regulatory expectations tighten and client programs become more global and complex, CDMOs are increasingly evaluated by the robustness of their supply chain management as much as they are by their technical capabilities. Sponsors are looking for confidence that their therapeutics will be handled consistently and predictably, in full compliance, across all geographic regions.
An integrated supply chain delivers that assurance. It embeds quality, data integrity, and risk mitigation directly into the process rather than layering it on top. Integration also enhances resilience when disruptions (geopolitical, environmental, logistical, or otherwise) occur. A connected network allows for a rapid, coordinated response in these cases because every element of the supply chain is managed under the same system, allowing contingency plans to be implemented quickly while minimizing impact to timelines and maintaining product integrity.
Building for the Future
The role and importance of CDMOs in the overall CGT market is only expanding. As more advanced therapies move toward commercialization and globalization, clients will need their partners to manage greater complexity with speed and compliance. An integrated, single-vendor supply chain solution provides the foundation for that future.
It allows CDMOs to operate with greater agility, shifting seamlessly between programs and regions without sacrificing control. It creates operational continuity across the lifecycle of each therapy from clinical supply to commercial distribution. And it establishes the infrastructure needed to adapt to new modalities and technologies as well as compliance requirements.
Cryoport Systems supports CDMOs through this transformation, providing an end-to-end platform that connects cryopreservation, logistics, BioServices and biostorage, secondary packaging and labeling, and consulting all under a unified, compliant framework. It’s a partnership built on efficiency, transparency, and trust, designed to simplify the complex and strengthen performance across every stage of ATMP development and delivery.
Together, we build supply chains that are not only compliant and efficient, but that are resilient by design. This enables advanced therapies to reach patients safely and reliably. At the end of the day, an integrated supply chain is so much more than a logistics strategy, it’s how CDMOs turn precision into performance, and how Cryoport Systems is Enabling the Outcome™.


