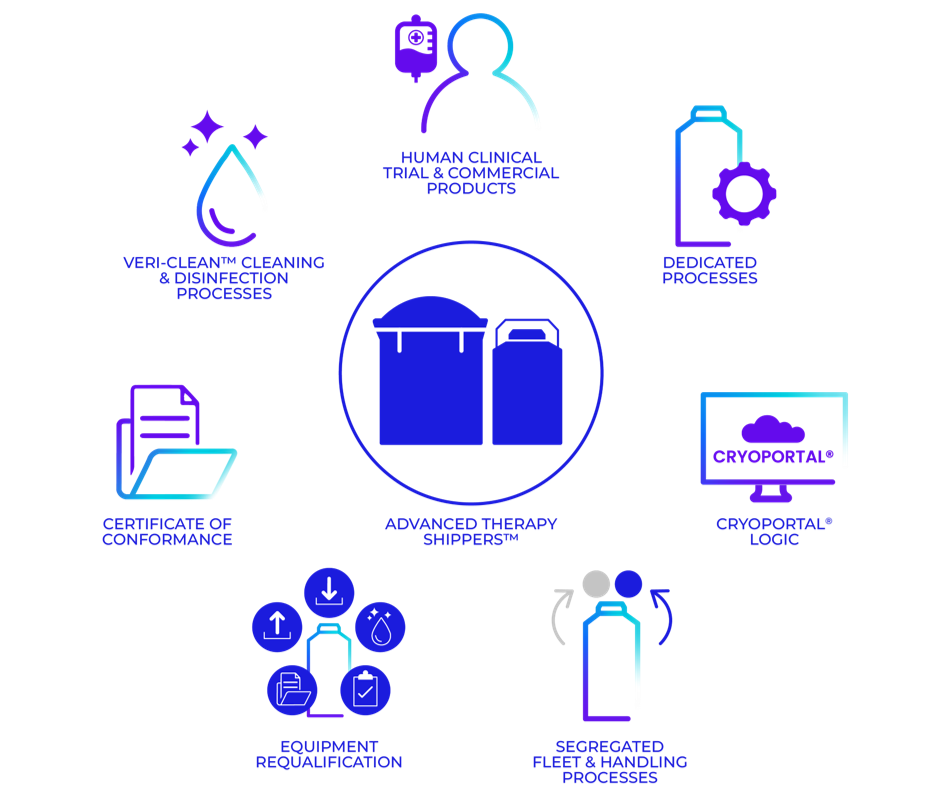
Advanced Therapy Shippers®
Ensuring Safe, Contaminant-Free Transport for Advanced Therapies
Dedicated Solutions for Critical Cell and Gene Therapies
Cryoport Systems offers Advanced Therapy Shippers® (ATS), shipping systems that are exclusively designed for the transportation of engineered human cell and gene therapies and human-derived cellular and biological materials. Our ATS fleet is segregated from our General Purpose (GP) fleet and developed to meet the highest standards of validation and decontamination, providing unmatched confidence in the safety and integrity of your critical therapies.
- Exclusive Use: ATS shipping systems are dedicated solely to human cell and gene therapies, segregated from GP shipping systems to prevent any possibility of cross-contamination.
- ISO 21973 Compliance: Designed to meet the newest ISO standards (ISO 21973) for the transportation of cells for therapeutic use.
- Unmatched Traceability: Full visibility of shipments through our Cryoportal® 2 Logistics Management Platform and Live View®.
- Rigorous Validation Protocols: Strict decontamination following our validated Veri-Clean® process guarantees pristine shipping environments.
- Integrated Chain of Compliance®: Seamless integration with our comprehensive Chain of Compliance® solutions for complete transparency.
A New Standard in Advanced Therapy Transportation
Advanced Therapy Shippers® Meet Evolving Regulatory & Market Demands
Uniquely Designed for Safer Transportation of Critical Patient Therapies
Our Advanced Therapy Shippers® are designed in response to growing market demand and anticipated regulatory requirements for safer transportation of critical patient therapies. By anticipating and addressing future regulatory requirements within the temperature-controlled supply chain, we ensure that our shipping systems and services remain at the forefront of innovation and compliance. Each shipper in our ATS fleet is engineered to eliminate the risk of cross-contamination, having never contained non-human-based materials. This ensures the integrity of your samples, aligning with the latest regulatory guidance and standards.
ISO 21973 Compliance for Human Clinical Trial & Commercial Products
The Advanced Therapy Shipper® guarantees each shipper has been used only for human commodities.
Cryoport’s Advanced Therapy Shippers® are restricted to use for only cell and gene therapies containing human clinical trial and commercial materials. This provides unmatched verification information and supply chain support for biopharmaceutical companies supporting advanced therapies. All of our shipping systems are meticulously designed to comply with the latest ISO 21973 standard, ensuring they meet general requirements for the transportation of cells for therapeutic use. This compliance guarantees that your shipments are handled with the utmost care and in line with industry best practices. Our processes are proven to maintain both Good Manufacturing Practice (GMP) and Good Distribution Practice (GDP) standards no matter the material type.
Segregated Fleet & Dedicated Processes
The Advanced Therapy Shipper® Fleet is Fully Segregated
Our Advanced Therapy Shippers® are their own fleet, segregated and handled separately from our General Purpose (GP) shipping systems in both process and physical separation. Each ATS shipping system is dedicated exclusively to human cell and gene therapies, ensuring they have never contained non-human-based materials. This commitment virtually eliminates the risk of cross-contamination, providing the highest level of confidence in the safety and purity of your therapies, as all ATS shipping components and materials maintain defined standards to ensure protection from cross-contamination.
For an additional layer of risk mitigation and protection, our Certificate of Conformance is a formal certification, signed by a quality assurance (QA) team member within Cryoport Systems, that certifies that every unique shipping system has only handled human cell and gene therapy products.
Cryoportal® 2 Logistics Management Platform
ATS Use is Managed by our Cryoportal® 2 Logistics Management Platform
Precision, reliability, and efficiency are non-negotiable within the intricate world of the life sciences. Cryoport Systems’ Cryoportal® 2 provides complete transparency and traceability of all equipment, components, and commodities while preventing violation of procedures or cross-contamination from order entry using programmatic logic that ensures ATS blue shipping systems are used exclusively for human advanced therapies.
Dewar Requalification and Chain of Compliance®
All Cryoport dewars are requalified after each use for physical suitability, cleanliness, LN2 capacity, and minimum required hold times.
As part of the requalification process, ATS shipping systems are reconfirmed to be of restricted use, only supporting cell and gene therapies containing human clinical trial and commercial materials. Our robust requalification process provides unmatched verification information and supply chain support for biopharmaceutical companies involved in the development and commercialization of cell and gene therapies.
Unlike conventional logistics solutions, Cryoport Systems offers unparalleled traceability, ensuring the integrity and custody of critical materials throughout their journey. Our Chain of Compliance® integrates validated requalification procedures, segregates human- and animal-derived products, and maintains a complete history of equipment performance and location. By leveraging extensive data collection and management capabilities, we mitigate risks and implement preventative measures to ensure the safe delivery of invaluable materials.
Veri-Clean™ Cleaning & Disinfection Processes
Veri-Clean® is the industry’s first and only validated cleaning and disinfection process.
Our custom and proprietary Veri-Clean® process virtually eliminates the risk of cross-contamination by decontaminating all of our shipping systems and stainless steel accessories. Validation elements include the initial bioburden evaluation of environmental flora present after the return of shipping systems (bacteria, fungi, and viruses), manual cleaning validation (protein and hemoglobin), and low-level disinfection validation (S. aureus, E. coli).
By partnering with Cryoport Systems, you are ensuring that your critical cell and gene therapies are transported safely, reliably, and in compliance with the highest industry standards. Learn more about how we are Enabling the Outcome™ for advanced therapies and supporting certainty through our risk-mitigating supply chain expertise — one patient, one therapy, one product at a time.