Expansive solutions for biologics distribution
Our comprehensive supply chain platform encompasses a full range of risk-mitigating solutions
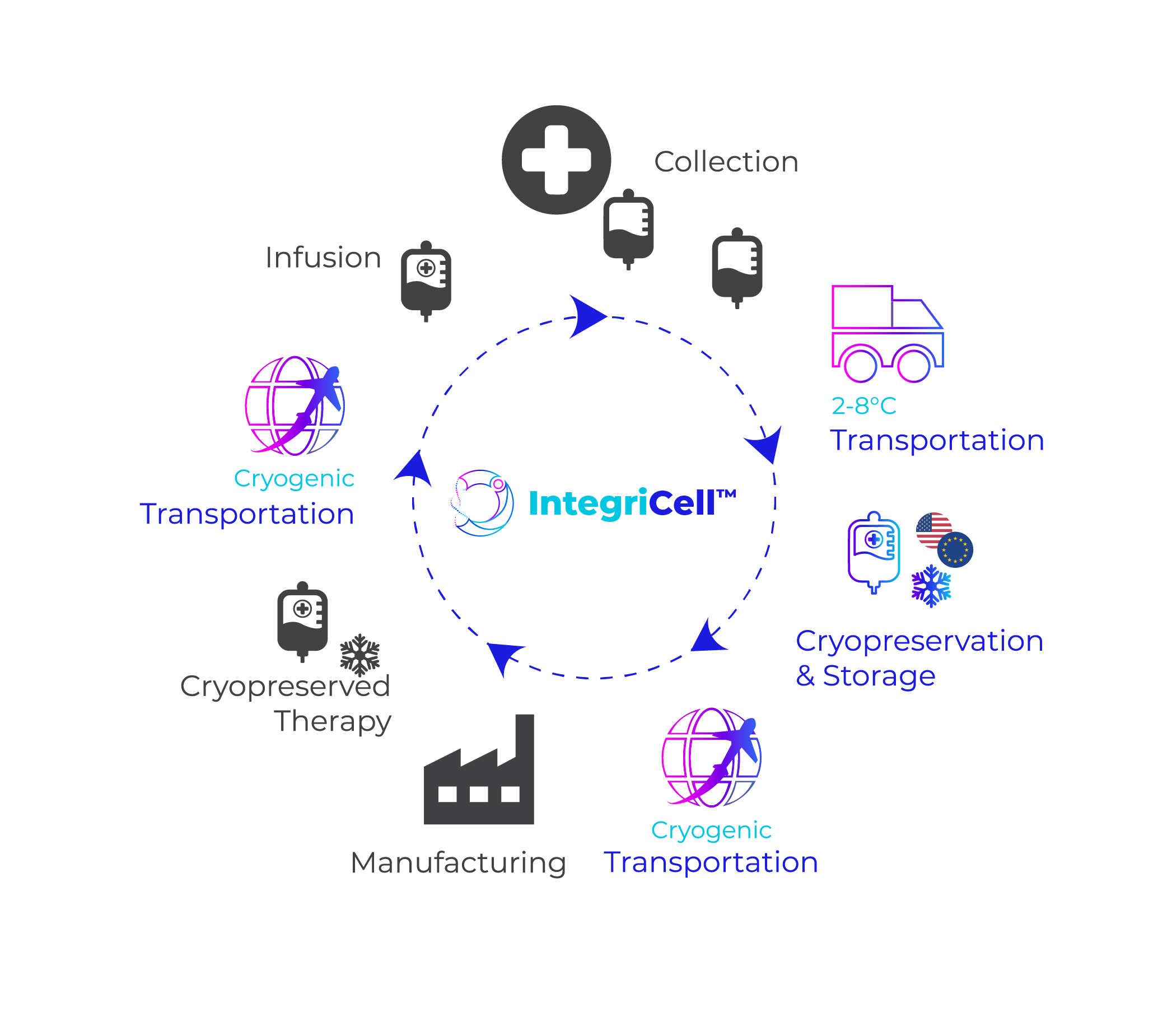
Distributing biologics requires meticulous planning and preparation. You must consider procedures like the cryogenic storage and transport of master cell banks, the bulk transfer of API (Active Pharmaceutical Ingredients), and the distribution of the products to the end user. Each of these steps require specific storage, packaging, and transportation considerations that are rarely accomplished in-house. We can help supplement your needs with our tailored services.
Devote more of your time and resources to your life-saving work
Cryoport Systems can manage the complexities of the biologics supply chain
At Cryoport Systems, our integrated platform of solutions can support you throughout the entire supply chain – from cryogenic storage and the transportation of master cell banks to -80°C transfer of API. We also offer direct-to-patient solutions through out sister company, CRYOPDP.
Fully-Integrated Supply Chain Platform
Cryoport Systems is a global supply chain leader with extensive expertise and capabilities to support the challenges of critical, high-value materials. Our fully-integrated supply chain platform offers comprehensive support throughout all stages and phases. With solutions that encompass BioServices and biostorage, cryopreservation, and tailored consulting services in addition to our industry-leading shipping systems and transportation services, our support scales alongside your biologics development.
Our platform is specifically designed to reduce risk. We offer the gold standard in quality, products, services, processes, and technologies that drive best-practices in meeting the requirements of evolving regulatory standards.
Strategic Partner of Choice
Cryoport Systems is the expert partner for advanced therapy programs and critical biologics backed by longstanding logistics excellence and our comprehensive platform of supply chain solutions. The path from scientific discovery to an approved therapy is lengthy and intricate, requiring close oversight of the supply chain at each step. Developers of novel treatments need a strategic partner with expertise in managing the specialized demands of biological therapies to help guide their programs to success.
Cryoport Systems offers an integrated yet agile supply chain platform of services and solutions, mitigating risks through every phase of development. With decades of experience enabling innovative life science breakthroughs, we understand the level of collaboration and forward-thinking needed to reach success. Our platform of products, services, processes, and technologies is designed to mitigate risk at every step and has been specifically focused on serving our biopharmaceutical clients as they overcome challenges within the temperature-controlled supply chain.
Focus on Quality
At Cryoport Systems, our longstanding dedication to quality and specific focus on the life sciences industry have made us a trusted leader in comprehensive temperature-controlled supply chain solutions. We have earned our credibility by successfully supporting hundreds of clinical trials and delivering over half a million shipments of sensitive, valuable, and often irreplaceable commodities across the globe.
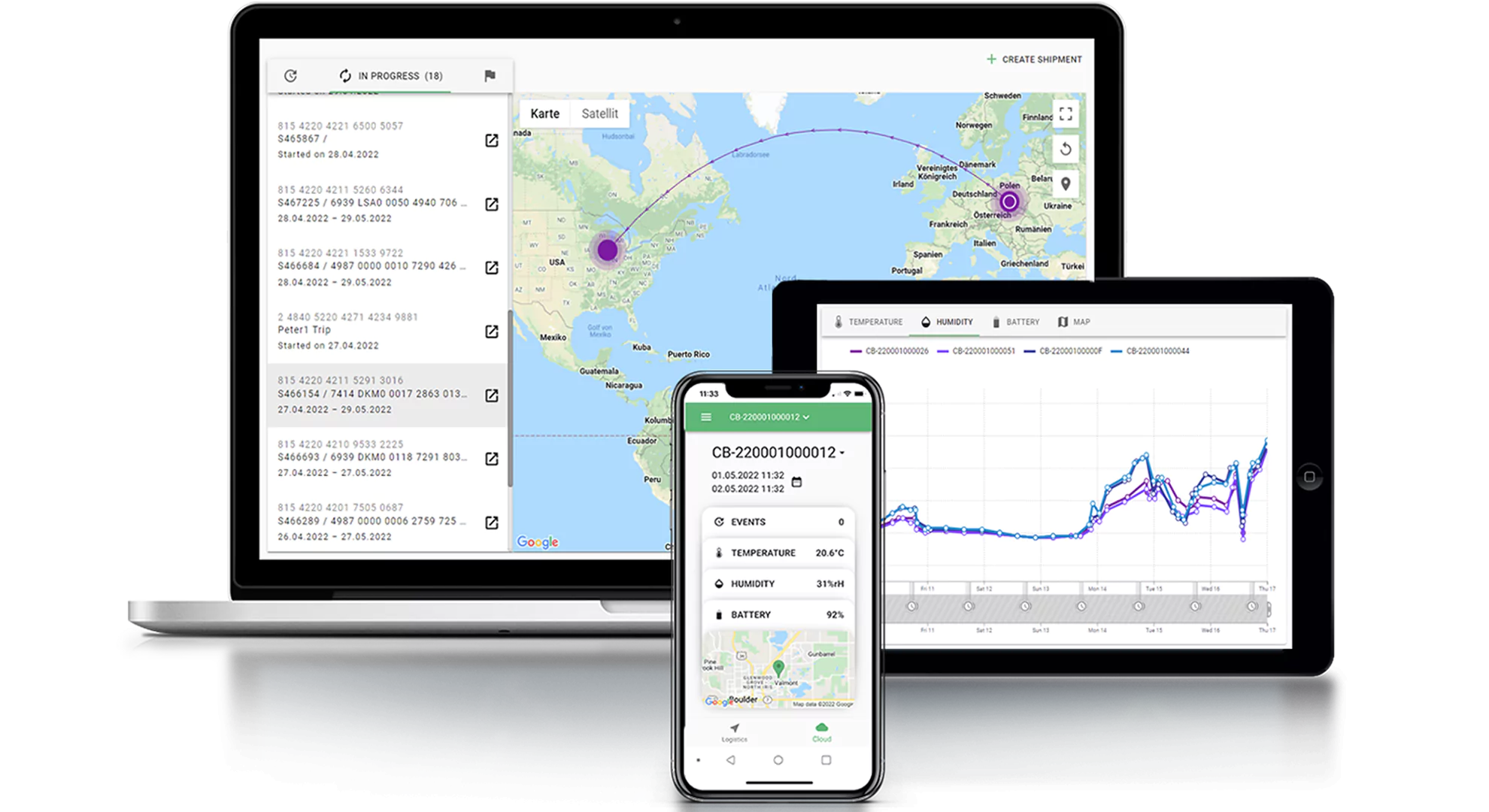
We drive best practices in the life sciences industry, and our experts have served as key contributors in developing ISO standards, including ISO21973, titled General Requirements for Transportation of Cells for Therapeutic Use. We work closely with ISTA, IATA, ASTM, and other internationally recognized and accredited bodies to design, develop, and execute verification and validation studies. We stand at the forefront of traceability in the biopharmaceutical supply chain, meeting GMP and GDP standards and playing a vital role in defining the compliance processes that will enable the next era of advanced medicine.
Scalability and Reproducibility
Our comprehensive supply chain platform improves and reinforces efficiencies in our clients’ systems and processes, building resilience into their strategic risk mitigation and contingency plans. Our BioServices and GMP depot equivalent Global Supply Chain Centers (GSCC) are strategically co-located to our world-renowned logistics to efficiently store, manage, and distribute critical materials with greater control. This effectively enforces risk-mitigating processes while reducing errors through a more efficient workflow, which ultimately provides optimized delivery methods to meet patient and client needs.
We support critical raw materials management and are equipped to handle cGMP starting materials and critical consumables. Our robust biopharmaceutical supply chain for temperature-controlled logistics includes the world’s largest cryogenic fleet of shippers, warehousing and fulfillment, and return logistics to overcome clients’ limited resources. We requalify every shipper, every time to ensure hold time, and use our validated Veri-Clean® cleaning procedures to avoid cross-contamination.
Cryoport Systems supports all phases of biological development from pre-clinical through to commercial. With strategic locations in the United States, Europe, and Asia, we are equipped to service global clinical and commercial programs.
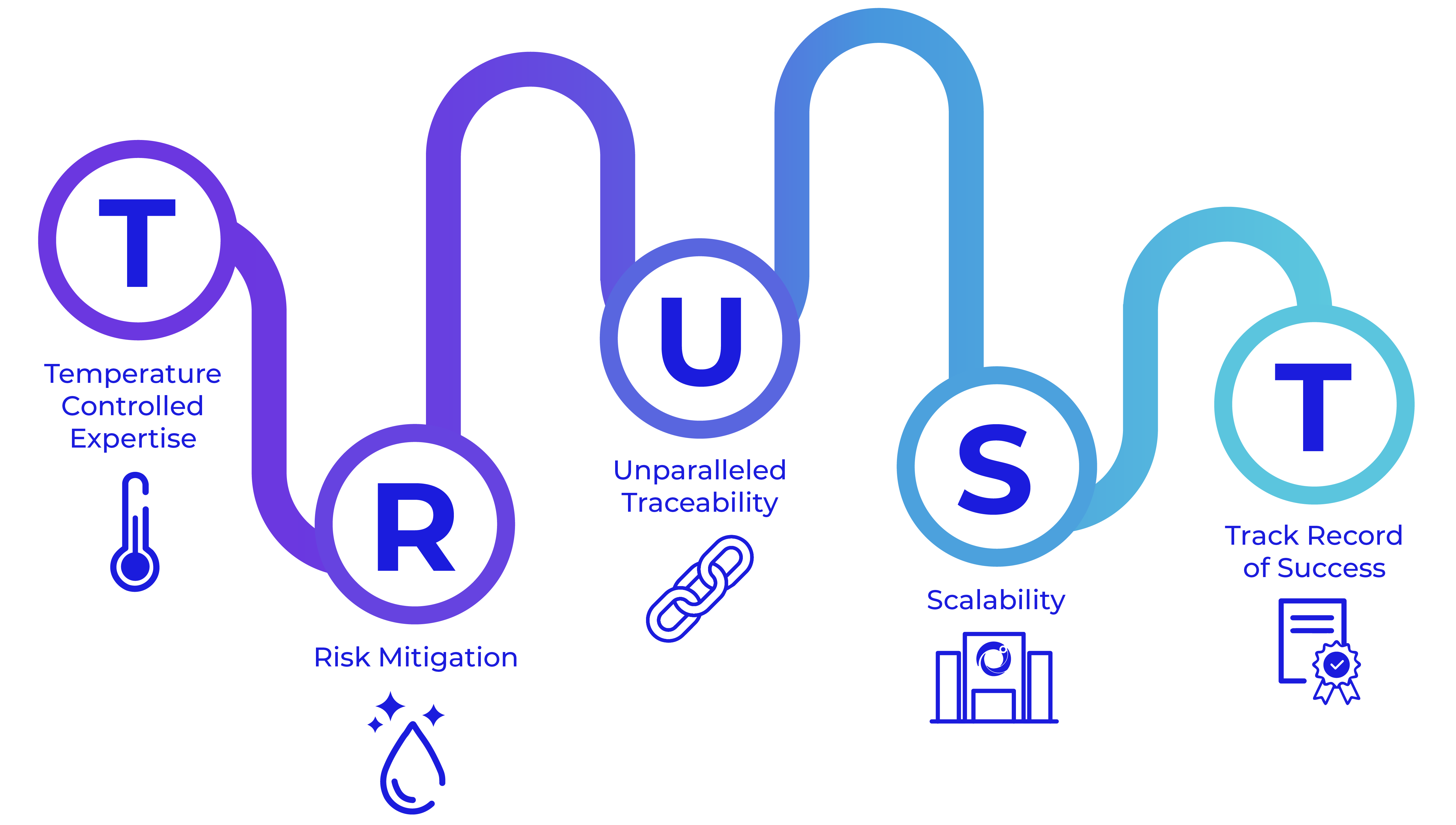
Cryoport Systems is Enabling the Outcome™ through our unparalleled supply chain platform.
Cryoport Systems was established by a team of doctors dedicated to serving the life sciences through expert logistics support. Today, our partnership capabilities have evolved beyond logistics into a robust platform of supply chain solutions that supports every stage and phase of the life sciences research, development, and manufacturing process. Our comprehensive platform of solutions makes Cryoport Systems a standalone vendor that can support all aspects of the temperature-controlled supply chain.
Learn More about how Cryoport Systems is Enabling the Outcome™