Supply Chain Archives

12/17/2025
2025 Year in Review: Turning Standards and Resilience into Outcomes
If you work within the advanced therapy ecosystem, you already know the story of 2025 from an industry standpoint, where science continued accelerating while funding and commercialization hinged on whether the supporting systems could keep up. For Cryoport Systems, that reality was a catalyst. This year, we raised the bar yet again and demonstrated how having the right strategic partnership behind your end-to-end supply chain can turn logistics into leverage, can transform compliance into confidence, and can spin scale into speed.
12/09/2025
What is ISO 21973 and Why It Matters for Cell and Gene Therapy
Cell and gene therapies (CGTs) are transforming medicine, but the complexity of these treatments introduces new challenges for supply chain management. Every shipment represents a therapy that could change or save a life, which is a level of responsibility that demands precision and transparency, as well as standards that leave absolutely no room for error. ISO 21973:2020 was created to meet that need.
10/30/2025
Cryoport Systems Sets New Benchmark for the Global Regenerative Medicine Supply Chain
Cryoport Systems has achieved a significant milestone by becoming the first global supply chain company worldwide to secure ISO 21973:2020 certification from the DNV Business Assurance. This certification marks a pivotal advancement in our mission to set the highest benchmarks for safety, traceability, and product integrity in the cell and gene therapy sector and across the life sciences.
10/28/2025
Built or Bought? Why Biotechs are Outsourcing Supply Chain Infrastructure
For companies developing advanced therapy medicinal products (ATMPs), building an in-house end-to-end supply chain may seem like the ultimate sign of maturity. But in today’s resource-constrained market, biotech leaders are increasingly asking themselves whether it’s better to build, or to buy. From startups operating on limited funding to global biopharma players scaling into new markets, outsourcing supply chain infrastructure is becoming more than just a cost saving tactic. By partnering with specialized providers, developers gain instant access to global networks, regulatory expertise and compliance, and technical capabilities that would take years and significant capital to build internally.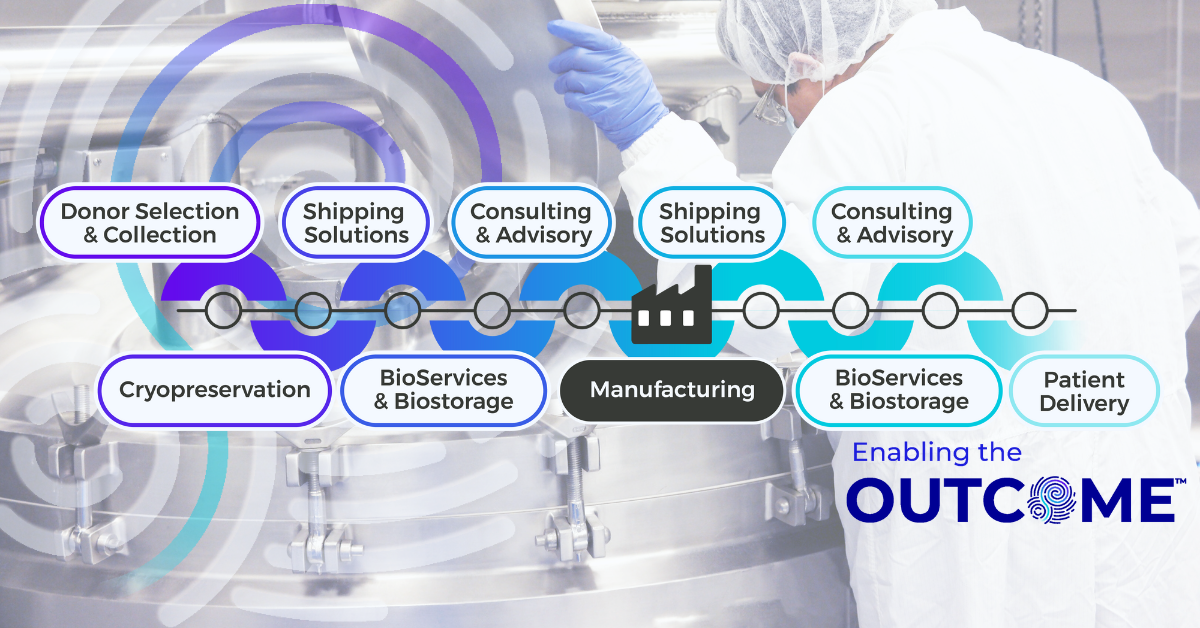
10/15/2025
The Connected Chain: Why Supply Chain Integration Matters in Advanced Therapy Manufacturing
Contract Development and Manufacturing Organizations (CDMOs) are at the heart of the advanced therapy ecosystem, managing complex, high-stakes programs that demand speed and compliance. As therapies move from early development into clinical trials and through to commercialization, every stage of manufacturing, packaging, biostorage, and final drug delivery need to align perfectly for seamless execution. Yet in many cases, these functions are managed by multiple vendors, each operating in isolation. The result is a fragmented supply chain that increases risk while slowing execution due to layers of operational burden.
09/10/2025
Scale Smarter: How Global Supply Chain Centers Amplify CGT Program Growth
Cell and gene therapy (CGT) programs are among the most complex and sensitive operations in the life sciences. From cryopreservation and biostorage to logistics and regulatory compliance, every step in the supply chain can influence product integrity, clinical timelines, and the scalability of the program. For program heads as well as clinical and technical operations teams, the challenge becomes bigger than merely moving materials. It quickly becomes a challenge of scaling efficiently while maintaining compliance and supporting growth across a global network.
08/29/2025
The Hidden Risks in Your Supply Chain: How Regulatory Support Can Save Your CGT Program
The temperature-controlled supply chain has become so much more than a straightforward logistics function, especially for advanced therapies. It has quickly evolved into a critical extension of your manufacturing process, your clinical trials, and your path to commercialization. Every step, from sourcing raw materials to delivering the final drug product to patients, must operate within strict regulatory and quality parameters where even the smallest oversight can cascade into regulatory delays, product loss, or even compromised patient safety.
08/04/2025
How Veterinary Practices Can Benefit from Reliable Logistics Partners
In veterinary medicine, success depends not just on skill and compassion, but also on having the right tools and treatments at the right time. From life-saving vaccines to advanced regenerative therapies, veterinary care today relies on a wide array of temperature-sensitive biologics. Ensuring these critical materials arrive safely and on time is no longer just a convenience, it’s a necessity.
07/30/2025
Beyond Logistics: Why Clinical Trial Success Depends on a Connected Supply Chain Strategy
Contract Research Organizations (CROs) are the orchestrators of modern clinical trials. From protocol development to site management and data collection, they serve as the hidden infrastructure that brings therapies from bench to bedside. But in the increasingly global and complex research landscape, a CRO’s ability to execute doesn’t just depend on clinical expertise, it hinges on the strength of the supply chain supporting every material movement and site milestone.
07/28/2025
Turning Risk into Resilience with Supply Chain Resilience for CDMOs
Contract Development and Manufacturing Organizations (CDMOs) are under more pressure than ever to deliver reliable results in an unpredictable world. From geopolitical instability to material shortages to increasingly complex regulatory expectations, the risks facing clinical and commercial programs have intensified. For CDMOs, these risks don’t just affect their own operations, they directly impact their clients’ ability to bring therapies to patients.Categories
- All (61)
- Industry Insights (20)
- Managing the Cold Chain (48)
- Navigating Logistics (27)
- Patient Access and Awareness (8)
Tags
- Asia
- ATMPs
- Biologics
- BioServices
- Biostorage
- CDMO
- CDMOs
- Cell Therapy
- Clinical Trials
- Cold Chain Management
- Commercialization
- CROs
- Cryopreservation
- Europe
- Fragmentation
- Gene Therapy
- Global Logistics Tracking
- Global Supply Chain Management
- International Shipping
- North America
- Risk Mitigation
- Standards and Compliance
- Supply Chain Management