Cryoport Systems’ Temperature-Controlled Supply Chain Blog
Learn more from our expert leaders on the latest industry trends, global news, and company updates.

Managing the Cold Chain
02/26/2026
Building Investor Confidence Through an Intentional Supply Chain Strategy
In early investment conversations, scientific innovation used to carry most of the weight. A compelling mechanism of action, a clear unmet need, and solid early data together were enough to advance the discussion and give investors a sense of potential. In today’s funding environment, however, that has shifted. Today, investors quickly move past the science and begin investigating whether a program can operate with the discipline and scalability required to grow.
Managing the Cold Chain
02/18/2026
Build Standardization Where It Matters Most: Turning Stability into True Consistency
There is a quiet assumption that many early-phase teams make when they transition to cryopreserved starting material... that freezing alone will solve their variability problem. On the surface, it feels true. Cryopreservation stops the clock, stabilizes the cells, and removes the minute-to-minute fragility of fresh material. But the stability that teams expect isn’t guaranteed by freezing itself, it’s created by how the freezing is done. And in practice, this is where programs often get surprised.
Managing the Cold Chain
01/27/2026
Establish Stability from the Start with Frozen Starting Materials for Cell Therapy Development
For many years, cell therapy programs defaulted to fresh leukapheresis-derived starting material. The assumption was that if you could minimize the time from collection to manufacturing, you would maximize viability. As a result, fresh apheresis became not only a scientific preference but an operational tradition, shaping how clinical teams would schedule donors, how manufacturing suites would allocate capacity, and how programs would structure day-to-day execution. Program leaders treated any deviation from “fresh” as a risk to be justified rather than a choice to be evaluated. But reality keeps getting in the way.
Managing the Cold Chain
01/27/2026
How Defragmenting the Pre-Clinical Supply Chain De-Risks Downstream Operations
Every stage of the supply chain including cryopreservation, logistics, and BioServices and biostorage, must align to ensure a seamless transition from pre-clinical work into Phase I trials. However, early-stage teams often select multiple vendors who each handle one piece of the process because it feels faster or more cost-effective in the moment. This approach, however, builds fragmented supply chains that introduce gaps as programs scale. And when those gaps surface, teams must devote valuable time and effort simply to get back on track.
Managing the Cold Chain
12/22/2025
Celebrating Innovation at the Cryoport Systems Grand Opening in Louvres, France
Cryoport Systems recently opened the doors of its newest Global Supply Chain Center (GSCC) in Louvres, France, marking a major step forward in how cell and gene therapies move safely and compliantly across Europe and the world. Strategically located just outside Paris and minutes from Charles de Gaulle Airport (CDG), this GSCC strengthens our integrated network and brings critical capabilities closer to sponsors, CROs, CDMOs, and clinical sites throughout the region, and strengthens our support of regional animal health and reproductive medicine organizations.
Industry Insights
12/19/2025
Expert Q&A: Is Standardization the Bottleneck or Breakthrough to Building Scalable and Cost-Effective CGT Programs?
Cell and gene therapy (CGT) companies are under constant pressure to do more while hitting milestones earlier. Demonstrate manufacturability. Ensure regulatory readiness. Design for scalability and patient access. And do all of this with more constrained resources and increasingly aggressive timelines. Building on our recent panel discussion, we gathered extended perspectives from Dr. Don Fink (Dark Horse Consulting Group), Audrey Greenberg (Mayo Venture Partners), Dr. Dominic Clarke (Cryoport Systems), and Kurtis Carlisle (Cabaletta Bio).
Industry Insights
12/17/2025
2025 Year in Review: Turning Standards and Resilience into Outcomes
If you work within the advanced therapy ecosystem, you already know the story of 2025 from an industry standpoint, where science continued accelerating while funding and commercialization hinged on whether the supporting systems could keep up. For Cryoport Systems, that reality was a catalyst. This year, we raised the bar yet again and demonstrated how having the right strategic partnership behind your end-to-end supply chain can turn logistics into leverage, can transform compliance into confidence, and can spin scale into speed.
Navigating Logistics
12/16/2025
Driving Compliance and Standardization in CGT Supply Chains
As cell and gene therapies (CGTs) continue to advance and more therapies reach late-stage clinical trials and commercialization, the complexity of the related logistics is only increasing. Therapies are crossing borders, reaching broader patient populations, and expanding into global distribution, all of which introduces risk if standards are inconsistent. Compliance and standardization, as a result, are essential for ensuring both scalability and patient safety.
Navigating Logistics
12/11/2025
Setting the Benchmark for Quality in Cell and Gene Therapy Logistics
Quality in cell and gene therapy (CGT) is the foundation that determines whether a therapy reaches a patient intact and effective. As the industry increasingly scales from early development of CGTs to global commercialization, the margin for error narrows as volume and complexity increase. Standards like ISO 21973 provide a framework, but true industry leadership comes from how those standards are applied. In this, Cryoport Systems is setting that benchmark.
Managing the Cold Chain
12/09/2025
What is ISO 21973 and Why It Matters for Cell and Gene Therapy
Cell and gene therapies (CGTs) are transforming medicine, but the complexity of these treatments introduces new challenges for supply chain management. Every shipment represents a therapy that could change or save a life, which is a level of responsibility that demands precision and transparency, as well as standards that leave absolutely no room for error. ISO 21973:2020 was created to meet that need.
Managing the Cold Chain
12/08/2025
Scaling for What’s Next in EMEA
As the advanced therapy medicinal products (ATMP) sector continues to expand across Europe and beyond, the infrastructure supporting these therapies needs to evolve just as quickly. Cryoport Systems is doing exactly that by scaling capabilities, deepening partnerships, and investing in new infrastructure to meet the growing needs of therapy developers.
Managing the Cold Chain
11/17/2025
How Global Shifts are Reshaping ATMP Logistics Beyond Borders
As advanced therapy medicinal products (ATMPs) continue to gain traction globally, the logistics required to move these therapies across borders are facing unprecedented challenges. From shifting trade policies to regional conflicts and Brexit legacy issues, global events are reshaping how therapies are distributed... and how supply chains must adapt in real time.
Managing the Cold Chain
10/28/2025
Built or Bought? Why Biotechs are Outsourcing Supply Chain Infrastructure
For companies developing advanced therapy medicinal products (ATMPs), building an in-house end-to-end supply chain may seem like the ultimate sign of maturity. But in today’s resource-constrained market, biotech leaders are increasingly asking themselves whether it’s better to build, or to buy. From startups operating on limited funding to global biopharma players scaling into new markets, outsourcing supply chain infrastructure is becoming more than just a cost saving tactic. By partnering with specialized providers, developers gain instant access to global networks, regulatory expertise and compliance, and technical capabilities that would take years and significant capital to build internally.
Navigating Logistics
10/22/2025
Beyond the Box: Why Shipping System Qualification Matters in Advanced Therapies
For advanced therapy logistics, quality isn’t limited to what happens inside the manufacturing suite. It extends across the entire supply chain, from packaging and transport to the systems that safeguard material integrity at every handoff. Yet one of the most critical (and often underestimated) steps in ensuring integrity is a shipping system qualification.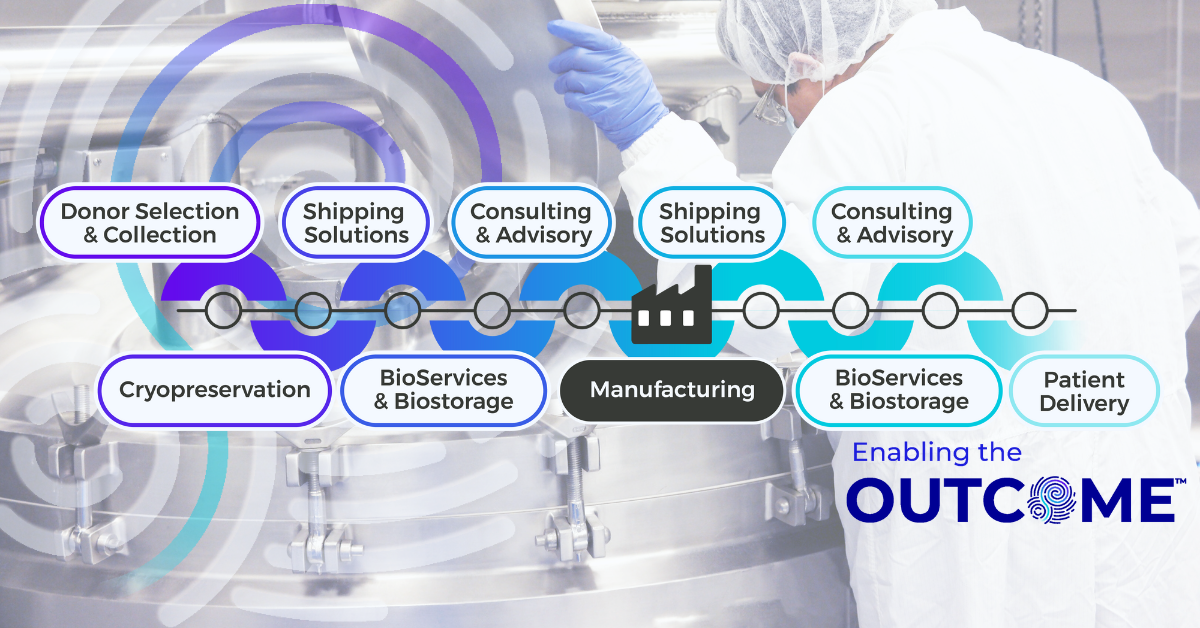
Managing the Cold Chain
10/15/2025
The Connected Chain: Why Supply Chain Integration Matters in Advanced Therapy Manufacturing
Contract Development and Manufacturing Organizations (CDMOs) are at the heart of the advanced therapy ecosystem, managing complex, high-stakes programs that demand speed and compliance. As therapies move from early development into clinical trials and through to commercialization, every stage of manufacturing, packaging, biostorage, and final drug delivery need to align perfectly for seamless execution. Yet in many cases, these functions are managed by multiple vendors, each operating in isolation. The result is a fragmented supply chain that increases risk while slowing execution due to layers of operational burden.
Managing the Cold Chain
10/08/2025
Resilience by Design: Lessons from Real-World Disruption
When it comes to Advanced Therapy Medicinal Products (ATMPs), disruption isn’t an “if,” it’s a “when.” When you’re dealing with unpredictable situations like geopolitical instability, sudden site closures, supply chain bottlenecks, or even weather-related delays, any disruption can potentially jeopardize patient treatments that may be irreplaceable. For sensitive therapies like these with narrow viability windows and strict temperature requirements, even a small deviation in process can have outsized consequences. In her Beyond Biotech podcast interview with Labiotech, Alison Pritchard, VP of Business Development for EMEA at Cryoport Systems, explained why resilience is one of the most critical (but often overlooked) pillars of ATMP delivery.
Supply Chain
10/01/2025
Cryoport Systems Redefines the Global Supply Chain with Launch of New Facility Near Paris, France
Cryoport Systems is thrilled to announce the grand opening of our revolutionary Global Supply Chain Center in Louvre, France. This cutting-edge campus is a one-stop location for end-to-end, temperature-controlled supply chain solutions and is designed to tackle the most complex challenges in the life sciences industry.
Managing the Cold Chain
09/15/2025
What’s Slowing the Journey from Therapy to Patient? Inside the Operational Hurdles of ATMPs
As more advanced therapy medicinal products (ATMPs) move from clinical trials to commercial launch, the industry has reached a critical inflection point. The science is advancing, approvals are accelerating, but patients still face steep barriers to access. In her Beyond Biotech podcast interview with Labiotech, Alison Pritchard, VP of Business Development for EMEA at Cryoport Systems, outlined the operational realities that stand between innovation and patient benefit. These challenges span manufacturing capacity, cold chain infrastructure, site coordination, and regional complexity.
Managing the Cold Chain
09/10/2025
Scale Smarter: How Global Supply Chain Centers Amplify CGT Program Growth
Cell and gene therapy (CGT) programs are among the most complex and sensitive operations in the life sciences. From cryopreservation and biostorage to logistics and regulatory compliance, every step in the supply chain can influence product integrity, clinical timelines, and the scalability of the program. For program heads as well as clinical and technical operations teams, the challenge becomes bigger than merely moving materials. It quickly becomes a challenge of scaling efficiently while maintaining compliance and supporting growth across a global network.
Managing the Cold Chain
08/29/2025
The Hidden Risks in Your Supply Chain: How Regulatory Support Can Save Your CGT Program
The temperature-controlled supply chain has become so much more than a straightforward logistics function, especially for advanced therapies. It has quickly evolved into a critical extension of your manufacturing process, your clinical trials, and your path to commercialization. Every step, from sourcing raw materials to delivering the final drug product to patients, must operate within strict regulatory and quality parameters where even the smallest oversight can cascade into regulatory delays, product loss, or even compromised patient safety.
Managing the Cold Chain
08/25/2025
What’s Next for ATMP Regulation in EMEA
For advanced therapy developers expanding into Europe, regulatory approval is just the beginning. Even after securing authorization from the European Medicines Agency (EMA), companies often find themselves mired in a patchwork of national rules, pricing hurdles, and even access delays. In her recent appearance on the Beyond Biotech podcast by Labiotech, Alison Pritchard, Vice President of Business Development for Cryoport Systems in EMEA, pulled back the curtain on what it really takes to bring advanced therapies to patients across the region.
Managing the Cold Chain
08/20/2025
Protecting the Foundation of Biologics with Flexible, Scalable Cell Banking
For any biotherapeutic program, your cell bank is the foundation of your manufacturing process. Master cell banks (MCBs), working cell banks (WCBs), and research cell banks (RCBs), represent years of development work and intellectual property. They are irreplaceable assets, and the security of these assets is directly tied to the long-term success of your program.
Managing the Cold Chain
08/04/2025
ATMP Development in 2025: Promise, Pressure, and the Push to Scale
As the advanced therapy medicinal products (ATMP) sector reaches the midpoint of 2025, there’s no shortage of scientific momentum. Groundbreaking CRISPR-based therapies are making headlines. Patient access to cell and gene therapies is expanding beyond early adopter markets. And a maturing global regulatory environment is slowly but surely creating new opportunities for commercialization. But alongside this promise comes pressure.
Managing the Cold Chain
08/01/2025
Built for Speed: How Cryoport Systems Delivers Without Compromise
In the life sciences, time isn’t just money, it impacts patient outcomes, regulatory compliance, and even trial integrity. When a temperature-sensitive shipment is delayed due to inventory shipper shortages or logistical bottlenecks, the impact can cascade across an entire program. Unfortunately, these scenarios aren’t theoretical. When you’re working with irreplaceable biologic materials, this kind of disruption quickly moves from inconvenient to unacceptable.
Managing the Cold Chain
07/31/2025
Earning the Next Trial: How Supply Chain Strategy Helps CROs Build Sponsor Trust
Contract Research Organizations (CROs) play a critical role in the biopharmaceutical industry, often executing clinical trials on behalf of sponsors. They’re tasked with wide-ranging expectations that span numerous functions, including maintaining the balance of operational excellence against regulatory compliance and cost efficiency. This complexity is compounded when clinical trial sites grow to span the globe, additional therapeutic areas, and expanded client populations. In such a competitive market, it’s not enough to simply deliver results. CROs must also build trust.
Managing the Cold Chain
07/30/2025
Beyond Logistics: Why Clinical Trial Success Depends on a Connected Supply Chain Strategy
Contract Research Organizations (CROs) are the orchestrators of modern clinical trials. From protocol development to site management and data collection, they serve as the hidden infrastructure that brings therapies from bench to bedside. But in the increasingly global and complex research landscape, a CRO’s ability to execute doesn’t just depend on clinical expertise, it hinges on the strength of the supply chain supporting every material movement and site milestone.
Managing the Cold Chain
07/29/2025
Helping CROs Strengthen Performance Through Smarter Supply Chain Strategy
The complexity of clinical trials only continues to rise, especially in areas like cell and gene therapy (CGT), where the stakes are higher and timelines tighter. Contract Research Organizations (CROs) are navigating this shift from the front lines, carefully managing operational timelines and supporting regulatory readiness while ensuring their sponsor clients receive high-quality, compliant outcomes across global sites.
Managing the Cold Chain
07/28/2025
Turning Risk into Resilience with Supply Chain Resilience for CDMOs
Contract Development and Manufacturing Organizations (CDMOs) are under more pressure than ever to deliver reliable results in an unpredictable world. From geopolitical instability to material shortages to increasingly complex regulatory expectations, the risks facing clinical and commercial programs have intensified. For CDMOs, these risks don’t just affect their own operations, they directly impact their clients’ ability to bring therapies to patients.
Managing the Cold Chain
07/25/2025
Scaling with Confidence for CDMO Supply Chain Alignment
Contract Development and Manufacturing Organizations (CDMOs) play a critical role in the life sciences industry, enabling biopharma innovators to bring new therapies from development to manufacturing to patients around the world. The demand placed on CDMOs has only increased in recent years, especially as cell, gene, and biologic therapies have moved from concept to clinic at unprecedented speeds. While the technical and scientific capabilities of CDMOs have advanced to meet these challenges, one area remains a frequent source of friction and delay...the end-to-end, temperature-controlled supply chain.
Managing the Cold Chain
07/24/2025
Building Better CDMO Supply Chain Readiness
With the accelerating growth of advanced therapies comes increasing complexity in the developing and manufacturing ecosystem. Contract Development and Manufacturing Organizations (CDMOs) sit at the center of this growth, providing critical manufacturing and development services on behalf of their clients, the therapeutic developers. CDMOs require the infrastructure and processes to efficiently scale production, maintain timelines, and ensure product integrity, all while navigating evolving regulatory expectations.
Industry Insights
07/22/2025
What’s Next for the CGT Industry? Mid-Year Check-In from the Leadership Team
We’re halfway through 2025, and the cell and gene therapy (CGT) landscape stands at a crossroads. Innovation continues to advance, yet the infrastructure required to support commercialization remains under pressure. To assess the current state of the industry and what’s ahead, Cryoport Systems gathered candid insights from our internal leaders across functions, from product development and commercial strategy to operations and client engagement.
Managing the Cold Chain
06/28/2025
Celebrating Logistics, the Unsung Hero in Cell and Gene Therapy Innovation
Every National Logistics Day offers a chance to recognize the systems and people that quietly power the innovation engine for Cell and Gene Therapy (CGT). While logistics frequently brings to mind images of trucks and warehouses, cardboard boxes, and tracking numbers, for CGT developers, it’s the infrastructure that protects innovation and ensures that time-sensitive, potentially life-changing treatments reach patients intact and on time. At Cryoport Systems, logistics has never been just about transportation. It’s about Enabling the Outcome™.
Navigating Logistics
06/27/2025
Bridging the Gaps: How Cryoshuttle® Enhances First and Last Mile Resilience
For advanced therapies, the most vulnerable parts of the transportation journey aren’t always the longest. In fact, the greatest risks often occur in the shortest distances. While intercontinental transport and customs clearance are well-known hurdles, both the first and last mile remain highly vulnerable to disruption. Cryoport Systems developed our Cryoshuttle® local pickup and delivery services to help close the gap between global transport lanes and local handling environments.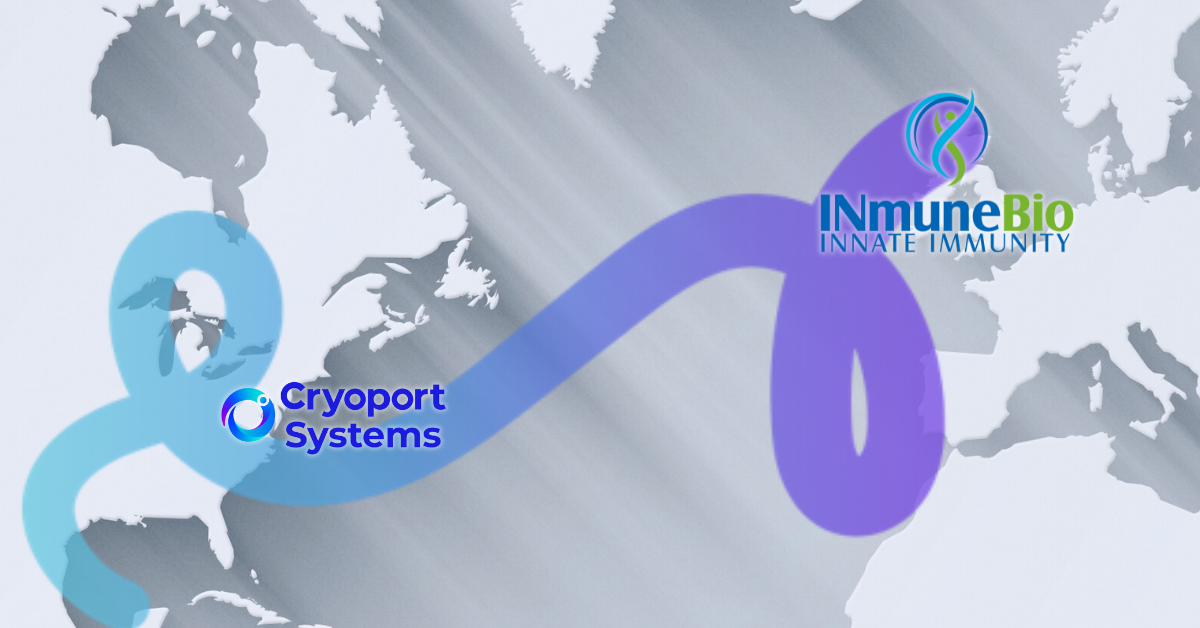
Managing the Cold Chain
06/24/2025
When Every Hour Counts: How Cryoport Systems Rapidly Mobilized to Protect INmune Bio’s Critical Clinical Materials
Biopharmaceutical development, particularly for advanced CGTs, relies on a robust infrastructure of logistics, BioServices, and temperature-controlled supply chains to ensure timely and intact delivery of drug products under stringent regulations. INmune Bio Inc. faced a challenge when a sudden biostorage site closure jeopardized a key clinical trial. However, with a strong support network, they turned to established partners like Cryoport Systems to overcome the crisis.
Patient Access and Awareness
06/19/2025
Supporting Access Through Supply Chain Excellence on World Sickle Cell Day
World Blood Donor Day serves as a reminder of the life-saving impact of voluntary blood and cell donations. These donations, often behind the scenes and not always recognized, are essential to emergency and surgical medicine and increasingly play an important role in cell and gene therapies. At Cryoport Systems, we recognize the incredible value of donors. We also understand that moving these donations safely, reliably, and under the strictest conditions, is a critical link between compassion and cure.
Patient Access and Awareness
06/14/2025
Recognizing Donors and Advancing the Future of Cell Therapy
World Blood Donor Day serves as a reminder of the life-saving impact of voluntary blood and cell donations. These donations, often behind the scenes and not always recognized, are essential to emergency and surgical medicine and increasingly play an important role in cell and gene therapies. At Cryoport Systems, we recognize the incredible value of donors. We also understand that moving these donations safely, reliably, and under the strictest conditions, is a critical link between compassion and cure.
Managing the Cold Chain
06/13/2025
Expanding Clinical Trials Internationally in the ATMP Space
Expanding an advanced therapy clinical trial internationally is an exciting milestone but can be daunting for researchers and biotech teams. International trials involve challenges like country-specific regulations, Qualified Person (QP) release, and complex logistics. Early planning for these issues can help avoid delays and ensure progress.
Industry Insights
06/04/2025
Engineering for Impact: A Conversation with Mike Dybicz on the HV3 and Safepak Systems
When it comes to cell and gene therapy (CGT) logistics, innovation is about more than improving efficiency, it’s about safeguarding a patient’s only chance at treatment. We sat down with Mike Dybicz, Senior Vice President & Chief Product Development Officer, to discuss how Cryoport Systems is reshaping the future of CGT logistics with innovative products such as Cryoport Express® Cryogenic HV3 Shipping System and Safepak® System 1800, designed specifically to reduce risk, increase reliability, and ultimately improve patient outcomes.
Patient Access and Awareness
06/01/2025
Honoring Survivors, Enabling the Future
Each year on the first Sunday in June, National Cancer Survivors Day serves as a powerful reminder that behind every innovation in medicine, every breakthrough treatment, and every clinical milestone, there are human stories of courage, resilience, and hope. At Cryoport Systems, we pause today not only to recognize the strength of cancer survivors but also to reflect on how our work supports the treatments that are changing survival outcomes.
Managing the Cold Chain
05/29/2025
Starting Smart: How to Structure an Integrated Supply Chain in Early-Stage CGT Programs
For many academic labs, technology spinouts, and early-stage CGT companies, the temperature-controlled supply chain feels like something to figure out later. But when dealing with highly sensitive starting materials and therapies like CGTs, later can quickly become too late. Whether you’re preparing for first-in-human trials or just beginning to plan for scale, integrated thinking now can spare you time, cost, and complexity later.
Patient Access and Awareness
05/19/2025
Clinical Trials Day: Supporting the Journey from Discovery to Delivery
Clinical trials represent hope, innovation, and the pursuit of better outcomes. They are the backbone of developing new diagnostics, vaccines, devices, and therapies. They help ensure safety, efficacy, and accessibility to the people who need them. That's why each year on May 20, the community comes together to celebrate Clinical Trials Day, honoring the anniversary of the first randomized clinical trial. At Cryoport Systems, we are honored to support our partners and clients in this journey.
Managing the Cold Chain
04/29/2025
Optimizing Cryopreservation for Leukapheresis: Advancing Cell and Gene Therapy Supply Chains
The supply chain for fresh leukapheresis is one of the most complex logistical challenges in cell and gene therapy (CGT). As a critical starting material, leukapheresis must be collected from donors, processed, and transported within a limited time window. These challenges increase variability and risk, ultimately impacting the success of advanced therapies. Alexandre Michaux, Process Development & MSAT Manager at IntegriCell™ , recently presented his work on the development of an automated cryopreservation process for leukapheresis in a Poster Presentation at Advanced Therapies Congress in London.
Industry Insights
04/22/2025
Advancing Standardization in Cell and Gene Therapy Supply Chains
The future of cell and gene therapy depends not only on scientific advancements but also on the efficiency and reliability of its supply chain. In a recent panel discussion at Advanced Therapies Congress in London, industry experts explored the critical need for standardization, the challenges in achieving it, and the opportunities to improve logistics, manufacturing, and delivery systems.
Industry Insights
04/18/2025
Transforming Patient Access to CGT: Q&A with Aruna Mor
Aruna Mor, Chief Commercial Officer at Cryoport Systems, recently delivered a powerful keynote at Advanced Therapies Congress in London, shedding light on the complexities of patient access and the critical steps needed to overcome these barriers. We sat down with her to delve deeper into some of the key themes of her keynote, exploring the intersection of science, logistics, supply chain, regulation, and collaboration that will determine the future of Cell and Gene Therapy (CGT).
Industry Insights
04/14/2025
Enabling the Outcome Through Enhancing Patient Accessibility
Cell and Gene Therapy (CGT) has emerged as one of the most transformative areas in modern medicine, shaping the future of healthcare and changing the face of next-generation therapeutic approaches. Yet, despite the continued scientific progress, a critical challenge persists in ensuring these therapies are accessible to the patients who need them most. This challenge was the focus of the keynote address at the Advanced Therapies Congress in London, delivered by Aruna Mor, Chief Commercial Officer at Cryoport Systems, where she spoke about to the state of patient access within the field of advanced therapeutics.
Navigating Logistics
03/24/2025
Strengthening Global ATMP Logistics: How Cryoport Systems’ Netherlands Facility Supports Temperature-Controlled Supply Chains
The success of ATMPs depends on more than just scientific breakthroughs, it requires a highly controlled, risk-mitigated supply chain that ensures product integrity from research through commercialization. With cell and gene therapies moving across multiple clinical sites, manufacturing facilities, and regulatory jurisdictions, developers need a logistics partner that can support seamless, temperature-controlled supply chain management at a global scale.
Managing the Cold Chain
03/21/2025
Strengthening ATMP Development in Europe: How BioServices Centers in France Enable Efficiency and Scalability
As Advanced Therapy Medicinal Product (ATMP) developers progress from early-stage research to late-phase clinical trials and commercialization, biostorage, sample management, and regulatory-compliant logistics become increasingly complex. Efficiently managing these critical components is essential to ensure product integrity, regulatory compliance, and seamless supply chain operations.
Managing the Cold Chain
03/21/2025
Optimizing ATMP Cryopreservation: How IntegriCell® Services in Belgium Ensure Cell Viability and Supply Chain Integrity
Cryopreservation is quickly becoming a critical yet often underestimated component of the Advanced Therapy Medicinal Product (ATMP) supply chain. The success of cell-based therapies depends on maintaining cell viability, stability, and consistency, yet variability in cryopreservation methods has compromised therapeutic outcomes.
Managing the Cold Chain
03/12/2025
Global Experts, Local Presence: Strengthening Europe’s Advanced Therapy Supply Chain
The development and commercialization of Advanced Therapy Medicinal Products (ATMPs) in Europe require a highly coordinated supply chain. From temperature-controlled logistics and biostorage to regulatory compliance, every stage of the ATMP lifecycle depends on precision, reliability, and risk mitigation
Navigating Logistics
03/12/2025
The Gateway to Europe: How Cryoport Systems’ Stevenage Supply Chain Hub Supports ATMP Development
For UK-based Advanced Therapy Medicinal Product (ATMP) developers, access to European clinical trial sites and commercial markets is a critical component of a successful growth strategy. Yet, navigating regulatory barriers, customs challenges, and supply chain complexities can create unnecessary delays, increase costs, and put the integrity of sensitive biologics at risk.
Navigating Logistics
03/04/2025
How Our Recent Innovative Solutions Meet Today’s Supply Chain Challenges
When advanced therapy organizations place innovation at the center of their approach to solving supply chain challenges, they prioritize the most important person involved – the waiting patient. An innovative, patient-centric approach reframes obstacles to focus on solutions that can ensure the vitality and protection of sensitive materials throughout every stage and phase of the journey to the patient’s bedside.
Industry Insights
02/12/2025
Ensuring Patient Impact in Advanced Therapy Logistics: A Conversation with Cryoport Systems’ Experts
The logistics behind advanced therapies aren’t just about moving shipments from point A to point B, they’re about protecting the integrity of life-saving treatments and ensuring that patients receive them without compromise. Following their discussion at Advanced Therapies Week 2025, we sat down with Aruna Mor, Chief Commercial Officer, and Mike Dybicz, Senior Vice President & Chief Product Development Officer, to dive deeper into how Cryoport Systems prioritizes patient impact at every step of the supply chain.
Patient Access and Awareness
02/12/2025
Advancing Cell and Gene Therapy on International Childhood Cancer Day
Every year on February 15th, International Childhood Cancer Day (ICCD) serves as a global platform to raise awareness about childhood cancer, advocate for improved access to care, and highlight the need for continued research and innovation. Pediatric cancer remains one of the leading causes of death among children worldwide, with approximately 400,000 new cases diagnosed each year.
Industry Insights
02/06/2025
The Patient Ferris Wheel: Centering the Patient in the CGT Supply Chain
In a recent interview at ATW, Cryoport Systems' leadership discussed how securing the future of medicine starts with minimizing risk and maximizing patient impact. Their conversation, The Patient Ferris Wheel – Centering the Patient in CGT Supply Chain, explored how Cryoport Systems is driving innovation in shipping systems designed specifically for advanced therapies.
Industry Insights
02/04/2025
Advancing Patient Outcomes Through Integrated Supply Chain Solutions
The landscape of advanced therapies is consistently shifting, requiring a seamless, high-quality supply chain to ensure the safe and effective delivery of life-saving treatments. In a recent interview at Advanced Therapies Week, Cryoport Systems leaders shared insights into how their integrated approach is shaping the future of supply chain management for the biopharmaceutical industry.
Patient Access and Awareness
02/04/2025
World Cancer Day: Advancing the Fight with Cell and Gene Therapies
Each year on World Cancer Day, the global community comes together to raise awareness, encourage prevention, and support those affected by cancer. It is a day of reflection, a time to recognize the immense progress that has been made in cancer research while acknowledging the work that still lies ahead.
Industry Insights
01/30/2025
Mapping the Future: Q&A with Mark Sawicki, Ph.D., on 2025 Trends in Cell and Gene Therapy
As the CGT industry enters a pivotal phase of commercial growth and therapy expansion, Cryoport Systems’ CEO, Mark Sawicki, Ph.D., shares his perspectives on where the market is headed.
Industry Insights
01/13/2025
Top 10 Industry Predictions for 2025
Cryoport Systems’ CEO, Mark Sawicki, provides a comprehensive analysis into the challenges and growth opportunities shaping the CGT industry.
Navigating Logistics
01/06/2025
The Future of Gene Therapy Logistics – A Pivotal Moment
Gene therapy is set to transform healthcare, but its success depends on logistics systems that can adapt and scale in line with the industry’s growth. Cryoport Systems is proud to support this exciting frontier with logistics solutions that prioritize product integrity, security, and compliance.
Navigating Logistics
12/30/2024
Supporting Compliance and Reducing Risk in Gene Therapy Logistics
When it comes to high stakes logistics in the gene therapy sector, adherence to regulatory standards isn’t just a box to check—it’s essential to delivering therapies that maintain integrity from production to patient. Cryoport Systems understands the unique risks in this space and has built compliance and risk mitigation into the very design of the Cryoport Elite™ Ultra Cold shipping system.
Navigating Logistics
12/23/2024
Meeting Market Needs through Collaboration and Customization
At Cryoport Systems, we understand that meaningful innovation comes not only from advanced technology but also from deeply collaborative relationships with our clients. Our collaborative approach has driven development of next-generation shipping systems and supply chain solutions, leaning on real-world insights from our clients to shape features, functionality, and future advancements.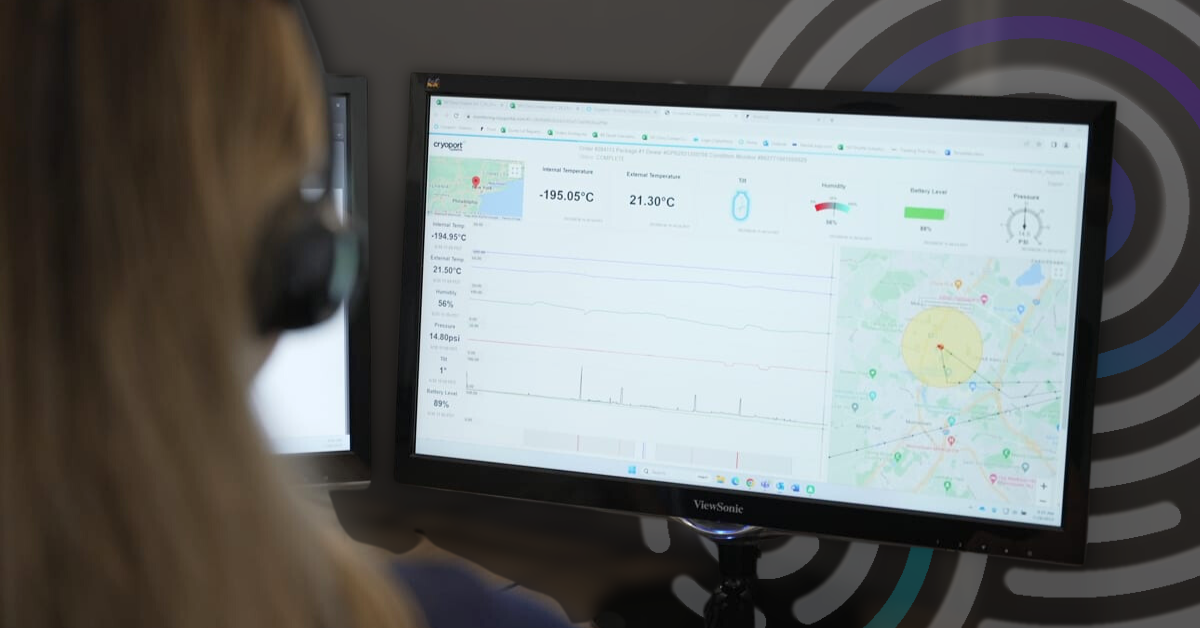
Navigating Logistics
12/16/2024
Solving Key Gene Therapy Logistics Challenges with Advanced Technology
Cryoport Systems developed the Cryoport Elite™ Ultra Cold shipping system with one mission in mind: to ensure that gene therapies reach their destination intact, uncompromised, and ready for patient treatment.
Industry Insights
12/11/2024
Reflecting on 2024: A Year of Groundbreaking Achievements
As we close out 2024, we reflect on a year marked by transformative milestones and significant growth at Cryoport Systems. This year, we took bold strides to redefine the future of supply chain solutions for life sciences.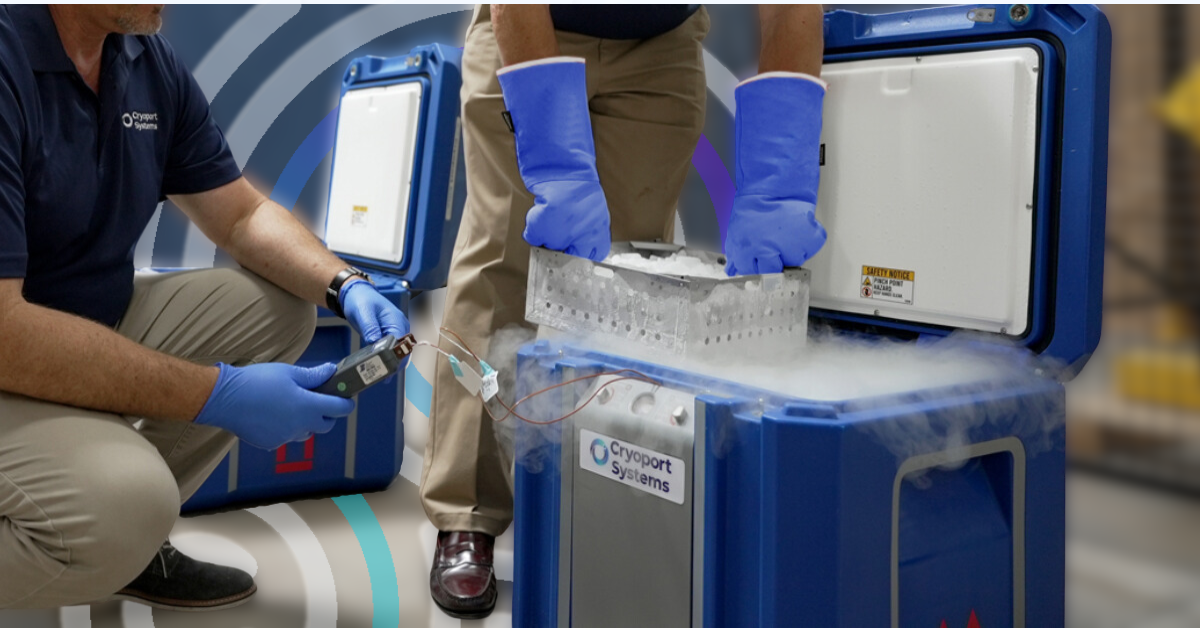
Navigating Logistics
12/09/2024
Meeting the Challenges of Gene Therapy Logistics with the Cryoport Elite™ Ultra Cold
As gene therapies move from research labs into clinical trials and commercialization, the need for secure, reliable logistics at scale is becoming an increasingly urgent need. Transporting these therapies requires a specialized approach.
Patient Access and Awareness
11/26/2024
National Family Health History Day: CGT Advancements for Generational Health
Since 2004, Thanksgiving Day in the U.S. has been paired with another lesser known but equally family-centric celebration – National Family Health History Day.
Navigating Logistics
10/04/2024
Certify Safety for Human-derived Materials with Advanced Therapy Shippers®
The risk of contamination can compromise life-saving therapies, making it a major concern within the world of transporting precision medicines.
Industry Insights
09/24/2024
Celebrating Our Recent Facility Expansion: A Recap of our Stevenage Global Supply Chain Hub Grand Opening Event
We recently had the pleasure of celebrating the official grand opening of our newest Global Supply Chain Hub in Stevenage, UK, alongside local clients and partners.
Navigating Logistics
09/03/2024
Cryoshuttle® Service Brings Local Support to Stevenage, England
In June, Cryoport Systems officially launched our newest Global Supply Chain Hub in Stevenage, England, a renowned center for cell and gene therapy development within the U.K.
Managing the Cold Chain
08/26/2024
3 Fundamental Services for Superior Master Cell Bank Management
The consistency of controlled conditions is vital throughout every step in producing advanced therapies, and the process usually begins with the secure handling and storage of the cells used for manufacturing.
Managing the Cold Chain
07/16/2024
IntegriCell®: Extending Cell Viability with Manufacture-ready Cryopreserved Leukopaks
During the COVID-19 pandemic, the restrictions on transportation and travel negatively impacted patients' access to life-saving treatments and support.
Managing the Cold Chain
06/27/2024
Cryoport Systems Celebrates Expanded French Facility with Ribbon Cutting Ceremony
Cryoport Systems commemorated the significant expansion of its Pont-du-Château facility in France with a ribbon cutting ceremony that officially launched the updated site.
Navigating Logistics
06/25/2024
The Cryoport Elite® Shipping System with Mike Dybicz, Cryoport Systems’ SVP & CPDO
In 2023, Cryoport Systems announced the launch of the Cryoport Elite™ shipping system.
Navigating Logistics
06/12/2024
Standardizing Compliance for Cell and Gene Transport with ISO 21973
It’s no secret that the cell and gene therapy market is one of the top-grossing markets within the life sciences industry.
Navigating Logistics
05/20/2024
Cryoport Elite® Shipping System: Industry-leading Advanced Therapy Support
The life sciences industry requires a robust shipping system that can handle the risks associated with transporting high-value commodities such as advanced therapies.
Managing the Cold Chain
04/16/2024
Overcoming Industry Obstacles for Cell and Gene Therapy Development
In the rapidly advancing world of cell and gene therapy (CGT) development, finding the proper solution to overcome common industry obstacles is a challenge that can impact entire production timelines.
Managing the Cold Chain
04/09/2024
National Holiday Facility Closures
As we look to the rest of 2024, Cryoport Systems wants to ensure that you’re up to date on our service schedule.
Navigating Logistics
03/27/2024
Tec4med: The Newest Addition to Cryoport’s Team
Cryoport Systems is a part of a nexus of supply chain solutions companies all under Cryoport, Inc. The other business units are CRYOGENE, CRYOPDP, and MVE Biological Solutions...
Industry Insights
02/22/2024
Examining 2023 & 2024: What’s to Come with President & CEO, Mark Sawicki
The cell and gene therapy industry is poised for many changes in 2024. With persistent challenges facing manufacturers and developers such as the retention of experienced talent or a general lack of funding for specialized therapies, the state of what’s to come feels more unsure than it has in recent years.
Managing the Cold Chain
02/06/2024
Enabling the Outcome with Cryoport Systems
Cryoport Systems entered the market over two decades ago to support the logistics processes of life sciences organizations. As industry demands evolved, our logistics capabilities expanded into a robust platform of supply chain management offerings.
Industry Insights
12/21/2023
Examining 2023 & 2024: Looking Back with President & CEO, Mark Sawicki
2023 was a year of highs and lows for the cell and gene therapy (CGT) market. The Alliance for Regenerative Medicine reported...
Managing the Cold Chain
11/14/2023
3 Services Your Biopharmaceutical Supply Chain Partner Should Offer
When it comes to choosing a cell and gene therapy shipping partner, not all providers are created equal.
Navigating Logistics
10/10/2023
The Crucial Role of Planning Ahead in Your Supply Chain Management
In the dynamic and ever-evolving realm of regenerative medicine and advanced therapies, the reliability of your supply chain could be the difference between success and failure.
Navigating Logistics
09/11/2023
4 Capabilities that Support a Superior Condition Monitoring Solution
For over 10 years, Cryoport Systems has been utilizing our near real-time condition monitoring solution that offers precise location information on shipments.
Navigating Logistics
08/16/2023
How Our Requalification Process Maintains Material Integrity
How can you be sure that your supply chain management partner understands the importance of cleanliness in standardized shipping practices?
Managing the Cold Chain
07/26/2023
A Glance into Our Houston & Morris Plains Global Supply Chain Centers
As cell and gene therapies continue to transform modern medicine, Cryoport Systems evolves its products and solutions to meet the needs of the industry and the patients it serves.
Managing the Cold Chain
05/31/2023
3 Services Your Biopharmaceutical Supply Chain Partner Should Offer
When it comes to choosing a cell and gene therapy shipping partner, not all providers are created equal.
Industry Insights
04/07/2023
Exclusive Q&A with Bruce McAfee, Director of BioServices Commercial Support
Cryoport Systems has extended its expertise in temperature-controlled supply chain solutions with vein-to-vein BioServices offerings.
Managing the Cold Chain
03/07/2023
Innovation and Risk Mitigation are Priorities for Cell&Co BioServices
At Cell&Co BioServices, a Cryoport Systems company, new product innovations are not adopted without significant testing. Recently our team dedicated 3 months to the creation and certification of a new type of cryogenic freezer technology.
Industry Insights
02/28/2023
State of the Industry: 2023 Predictions with Dr. Mark Sawicki
Cryoport Systems has grown to become a leader in the cell and gene therapy industry since its founding in 1999.
Industry Insights
12/13/2022
Documentary: Cell & Gene Therapy – Advancing The Next Generation of Pharmaceuticals
In this video documentary, American Pharmaceutical Review and Pharmaceutical Outsourcing magazine spoke with Cryoport Systems' CEO, Mark Sawicki...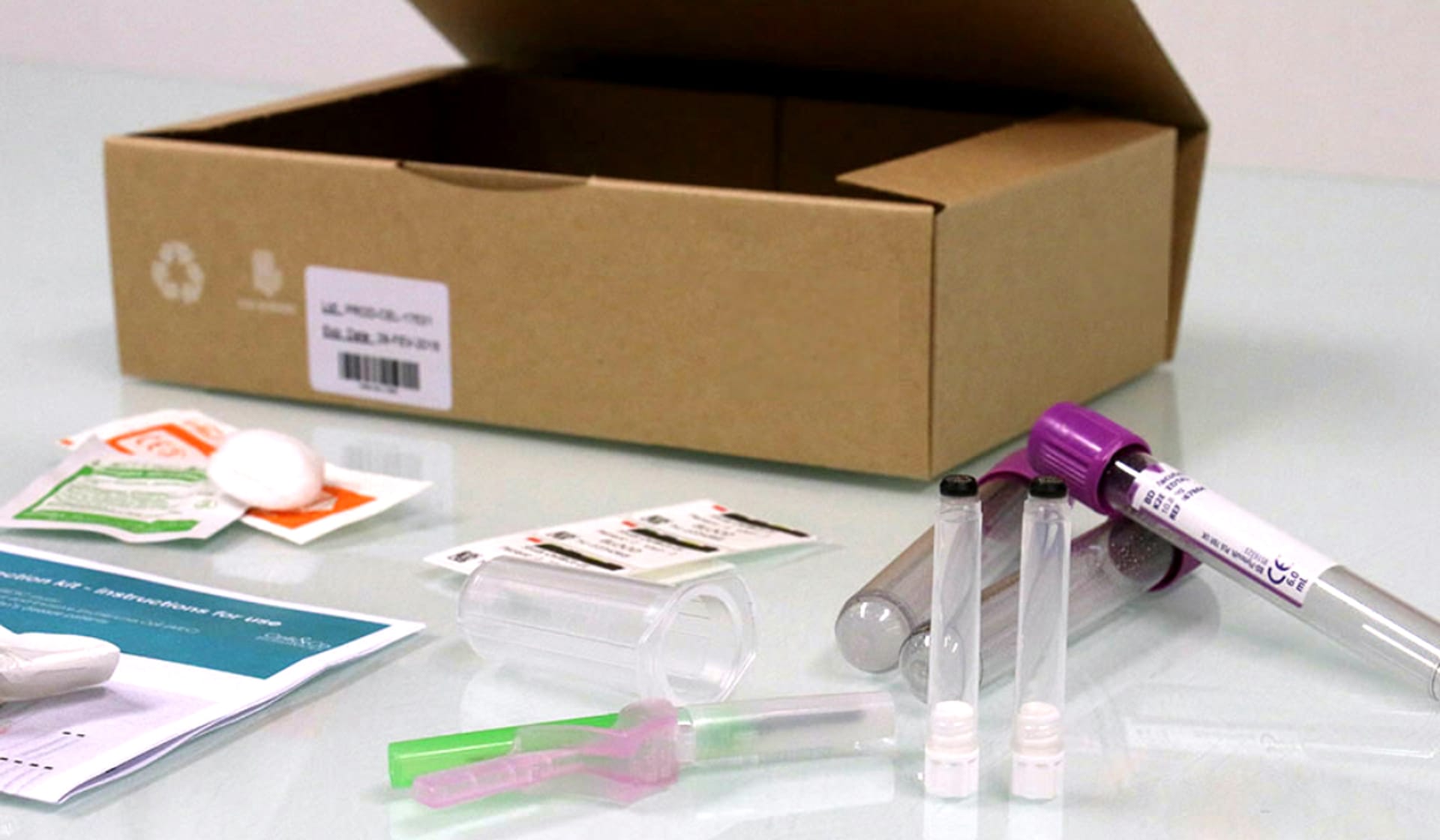
Managing the Cold Chain
11/30/2022
Through Our Newly Launched Global Supply Chain Centers, Cryoport Systems Now Offers Kit Production
Cryoport combines its expertise in temperature-controlled supply chain solutions to develop a comprehensive offering of vein-to-vein services, one of which includes Kit Production.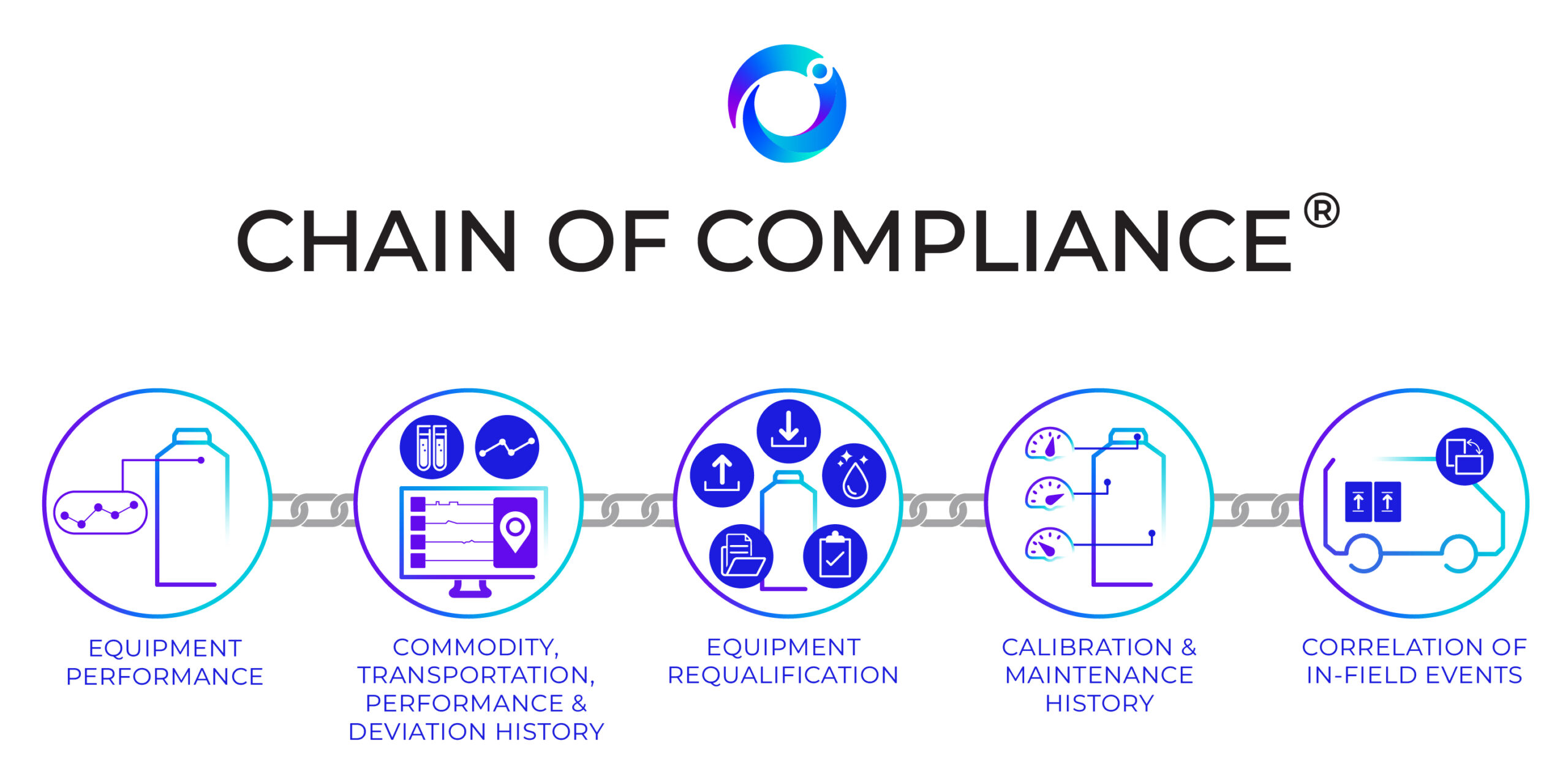
Navigating Logistics
10/19/2022
Cryoport’s Chain of Compliance®: Collects, Interprets, and Leverages Comprehensive Data to Enable a Significantly Smarter Supply Chain
Companies vying to be the first to market with breakthrough treatments have a great deal riding on the success of their efforts.
Navigating Logistics
10/13/2022
Cryoshuttle® Local Delivery and Pickup: Now Serving More Areas
Many biopharmaceutical companies, academic institutions, and manufacturing sites often need to move their valuable life-saving therapies and research materials locally for a variety of reasons...
Navigating Logistics
08/29/2022
The Cryoport Way: A Leap Forward in Standardizing the Regenerative Medicine Supply Chain
As cell and gene therapies are rapidly moving beyond clinical trials and into commercial markets, the safe and effective delivery of these life-saving treatments needs to be a priority for advanced therapy developers.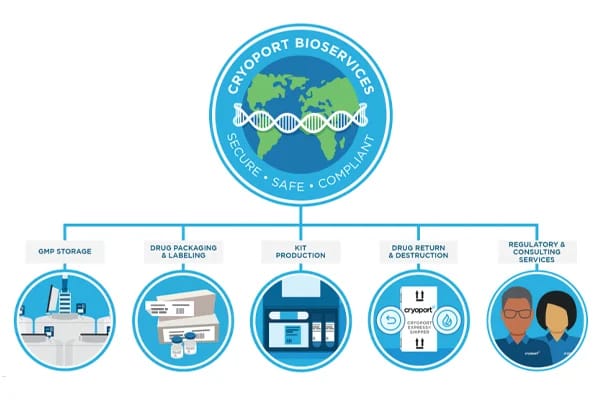
Managing the Cold Chain
08/22/2022
Cryoport Systems’ Global BioServices: Secondary Packaging and Labeling Solutions
Cryoport Systems’ highly anticipated Global Supply Chain Centers have been operational for a few months, and customers continue to move biopharmaceutical materials into our Houston, Texas...
Patient Access and Awareness
07/20/2022
The Untapped Potential for Cord Blood as Allogeneic Starting Material
In support of Cord Blood Awareness Month, Cryoport Systems’ partner, Be The Match BioTherapies®, led a webinar focused on the untapped potential and benefits of cord blood for prospective therapies.
Managing the Cold Chain
06/06/2022
Cryoport Systems Global Supply Chain Center: Ready for Launch!
We are quickly approaching the Grand Opening of our first two Global Supply Chain Centers in Houston, TX on June 14th and Morris Plains, NJ on June 16th, 2022.
Navigating Logistics
05/19/2022
New Product Launch: The Latest Enhancements to Cryoport Express® Standard & Combo Cryogenic Shippers
At Cryoport Systems, we are continuously striving to align with the latest science and technology advancements in our products and services, integrating the most advanced packaging, informatics...
Industry Insights
02/11/2022
Are You Ready for the Upcoming Data Network Transition? Here’s Everything You Need to Know
The future for global advanced therapy shipments requiring critical near real-time chain of custody, chain of condition and Chain of Compliance® reporting will soon face a significant technology challenge.Categories
- All (62)
- Industry Insights (20)
- Managing the Cold Chain (50)
- Navigating Logistics (27)
- Patient Access and Awareness (8)
Topics
- Asia
- ATMPs
- Biologics
- BioServices
- Biostorage
- CDMO
- CDMOs
- Cell Therapy
- Clinical Trials
- Cold Chain Management
- Commercialization
- CROs
- Cryopreservation
- Europe
- Fragmentation
- Gene Therapy
- Global Logistics Tracking
- Global Supply Chain Management
- International Shipping
- North America
- Risk Mitigation
- Standards and Compliance
- Supply Chain Management